Abstract
Mesothelioma is an uncommon cancer in dogs for which there is no established stan- dard of care. Chemotherapy is often suggested despite no definitive proof of efficacy. The aim of this study was to evaluate the impact of chemotherapy on survival of dogs with mesothelioma. A retrospective multicentric study was carried out. To be included, dogs needed to present an evocative clinical evolution and a morphological diagnosis of mesothelioma. Exclusion of other cause of effusion and complete clinical follow-up were also required. Fourty dogs were included, 27 received chemotherapy (group 1) and 13 did not (group 2). Groups were heterogeneous regarding the proportion of animals undergoing surgery as part of their treatment (16 in group 1, 2 in group 2; p = .016) and homogeneous otherwise. Univariate analysis showed that dogs from group 1 survived significantly longer than dogs from group 2 (MST: 366 vs. 74 days; p < .001). Complete resolution of effusion after the first chemotherapy administration positively correlated with survival in group 1 (MST: 415 vs. 160 days; p < .01). All other variable tested had no significant impact on survival in univariate analysis, but dogs undergoing surgery and dogs having serous membranes’ modification at medical imaging tended to survive lon- ger. Multivariate analysis confirmed that chemotherapy was the sole variable indepen- dently associated with survival in our study (odds ratio 5.57–6.12; p < .01).
KEYWORDS
dogs, drug therapy, mesothelioma
1 | INTRODUCTION
Mesothelioma is a rare neoplasm in dogs originating from mesothelial
cells. Its main clinical manifestation is effusions of the affected
Franck Floch currently works at AniCura TRIOVet, Rennes and Didier Lanore currently works at Hopia, Guyancourt, France.
coelomic cavities. Its diagnosis is often challenging. Indeed, neoplastic and reactive hyperplastic mesothelial cells can appear very similar, and it is difficult to distinguish one from another at cytopathological exam- ination in most cases.1,2 Histopathological analysis can help in making the difference in some cases: invasion of the underlying tissues and presence of distant or lymph node metastasis are suggested criteria to differentiate mesothelioma from reactive mesothelium.3 However,
presence of embolized mesothelial cells in lymph node has been reported in benign conditions and invasion into deeper structure can- not always be evaluated depending on the biopsy technique.4,5 Fur- thermore, absence of tissue invasion does not preclude the diagnosis of mesothelioma.3,6 Also, immunochemistry can help to comfort the mesothelial origin but it cannot demonstrate the neoplastic nature of the proliferation.6–8 Furthermore there is no validated marker or com- bination of markers that is very specific for mesothelioma.7–9 Conse- quently, morphological assessment alone, even with histopathology or immunochemistry, appears insufficient to diagnose mesothelioma; and, the final diagnosis is often the result of an integration of clinical, imaging and pathological data.
The absence of consensus on diagnostic procedures and the rarity of the disease may be partly responsible for the paucity of information regarding the therapeutic approach of canine mesothelioma. In peo- ple, mesothelioma is mainly pleural. It is also a rare and relatively poorly documented disease. Current therapeutic recommendations are based on surgical resection, when feasible, and platinum-based chemotherapy.10–12 In dogs, information on treatment is limited. Sur- gical procedures are often proposed in order to decrease the effu- sion’s consequences, and sometimes in a cytoreductive intent. For instance, pericardiectomy is commonly performed for management of pericardial mesothelioma, and pleural port device are increasingly used for the management of pleural mesothelioma. Though, outcome associated with these surgical procedures is poorly documented.13–16 Regarding systemic treatments, for years the rationale for use of che- motherapy was based on a tendancy of better survival of treated dogs in very small cohorts of dogs or isolated reports compared to historical data.1,17,18 Recently, a larger cohort of 34 dogs, including a control group, was published by Moberg et al. and also reported a better out- come in dogs with mesothelioma treated with chemotherapy.16 In this study, most dogs had pleural mesothelioma and the most used che- motherapeutic agent was 5-fluorouracil, administered intracavitarily. No survival prognostic factors were identified.
The primary objective of this study was to evaluate the impact of chemotherapy on survival of dogs with mesothelioma. The secondary objective was to determine prognostic factors associated with survival among treated dogs.
2 | METHODS
2.1 | Cell line validation statement No cell line was used.
2.2 | Case selection
Dogs diagnosed with mesothelioma between January 2004 and April 2020 were included from six veterinary institutions. To be included in the study, dogs needed to fulfil four inclusion criteria. First, a morpho- logical diagnosis of mesothelioma by a board-certified pathologist or
clinical pathologist was necessary. However, this was not considered sufficient in the absence of consensus on morphological diagnosis of mesothelioma. Second criteria was a clinical course evocative of mesothelioma, with recurrent effusions of no other possible cause identified. Third, medical imaging of the cavity involved needed to be performed at diagnosis. Fourth, a complete medical record and follow-up needed to be available for review.
2.3 | Data collection
For all animals, information collected regarding diagnostic procedures included review of cytopathological or histopathological report, neoplas- tic cavities’ imaging reports, and staging procedures. Result of immuno- staining were also collected when available. Double positivity for antibody-staining against cytokeratin AE1/AE3 and vimentin or smooth actin was considered suggestive of a mesothelial origin. Staging proce- dures included thoracic, cardiac and/or abdominal imaging associated with morphological analysis of any suspicious lesion identified. Imaging procedures included radiographs or CT scan for thorax, echocardiogra- phy for cardiac evaluation and, ultrasonography or CT scan for abdomen.
Regarding treatments, data collected included every therapeutic procedure performed or medication administered, including dose and frequency. Regarding chemotherapy, data collected included molecule, dose, frequency, route of administration (intravenous, intracavitary, or both), adverse events, response to treatment, and date of relapse when applicable. Adverse events were retrospectively classified according to VCOG-CTCAE.19 Response to treatment was evaluated based on persis- tence of effusion at sonography. When possible, evolution of measurable nodule or mass were evaluated according to the RECIST criteria.20,21 Regarding therapeutic surgery, it included any decompressive or cyto- reductive surgical procedures, such as pericardiectomy or pleural port placement. On the contrary, diagnostic surgical procedures (i.e., biopsy collection) were not considered a therapeutic procedure.
In addition, signalment (age, breed, gender and reproductive sta- tus), duration of clinical signs prior to diagnosis, number and location of neoplastic effusions at diagnosis, and survival time were also retrieved from medical files.
2.4 | Statistical analysis
Dogs were divided into two groups depending on the treatment they received. Dogs receiving chemotherapy constituted group 1 and other dogs constituted group 2.
First, homogeneity between groups was verified. Categorical data tested were gender, reproductive status, number and location of effu- sions, initial clinical signs duration (categorized as more or less than 30 days), presence of abnormalities of serous membranes at medical imaging and performance of surgery. For those, Pearson’s Chi-squared test was performed when there were more than five individuals in each set of data; if not, Fisher’s exact test was performed. Continuous data included age and clinical signs duration. Shapiro–Wilk test was
performed to assess their distribution type. Both were not normally distributed; therefore Mann–Whitney U test was used to assess the homogeneity between groups. Statistical significance threshold was set at p < .05 for all analyses.
The endpoint of the study was survival. It was defined as the dura- tion from the first morphological diagnosis to death from progression of the disease or censor. Dogs were censored if they were still alive at time of data collection, lost to follow-up or if they died from another cause. For survival analyses, duration of clinical signs was split into two groups of <30 days or ≥30 days and considered as a categorical data. Categorical data evaluated for their impact on survival included those previously mentioned and group. Age was the only continuous data evaluated. Comparison of survivals was performed using Kaplan–Meier method followed by log-rank tests for categorical data, and Pearson’s correlation product for continuous data. Significance was set at p < .05 for univariate analysis but all data with p < .20 were then included in a Cox proportional hazards model multivariate analysis.
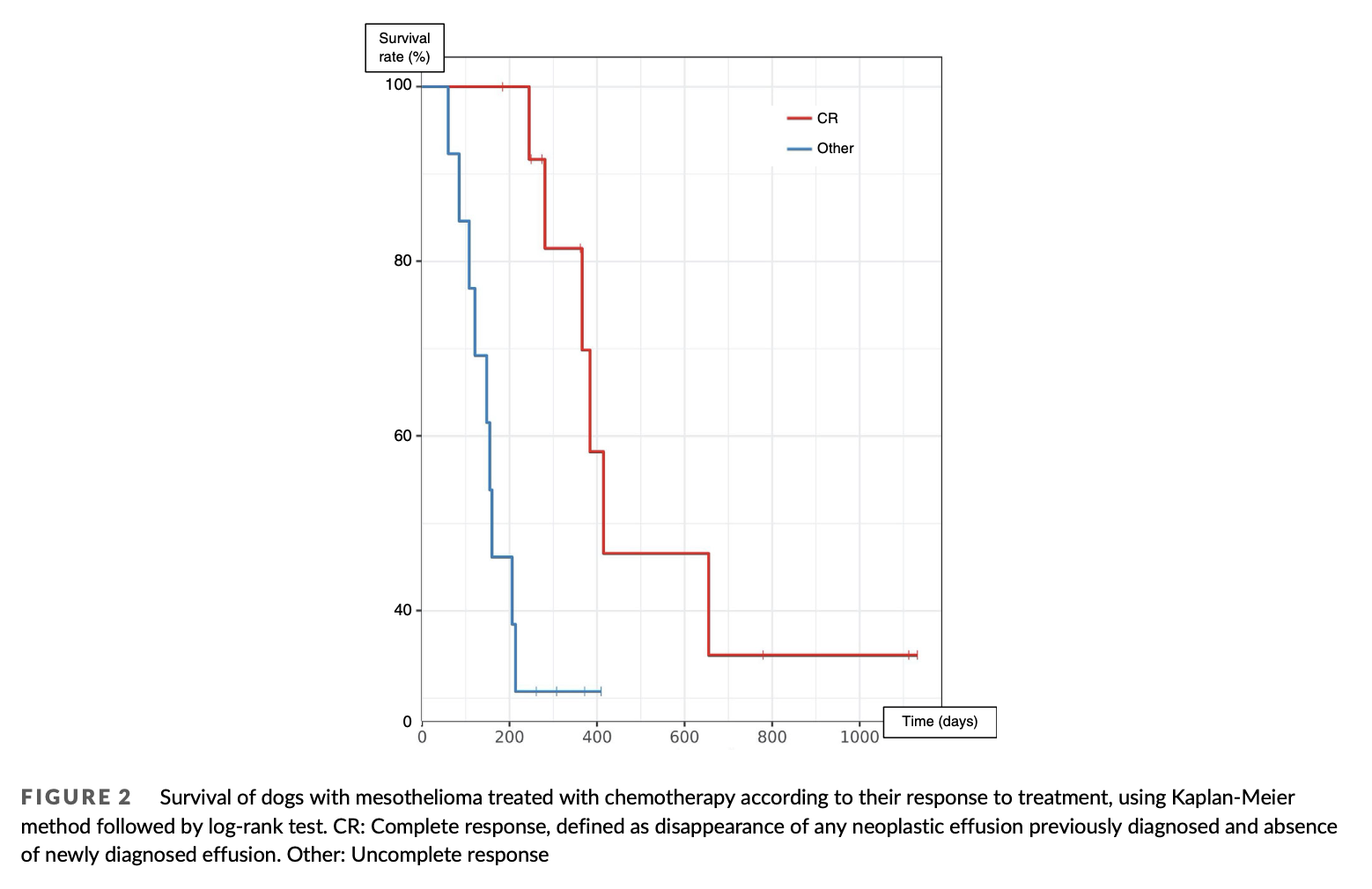
Another analysis was performed among group 1 to detect any rel- evant prognostic factor. Tested parameters included response to treatment, route of chemotherapy administration and all categorical data previously mentioned. Response to treatment was retrospec- tively classified into complete and uncomplete, based on ultrasonogra- phy performed 3weeks after the first chemotherapy session. Complete response (CR) was defined as disappearance of any neo- plastic effusion previously diagnosed, absence of newly diagnosed effusion. Progression-free interval (PFI) was evaluated only for com- plete responders and was define as the duration between the first documented CR to effusion relapse.
3 | RESULTS
3.1 | Population and clinical presentation
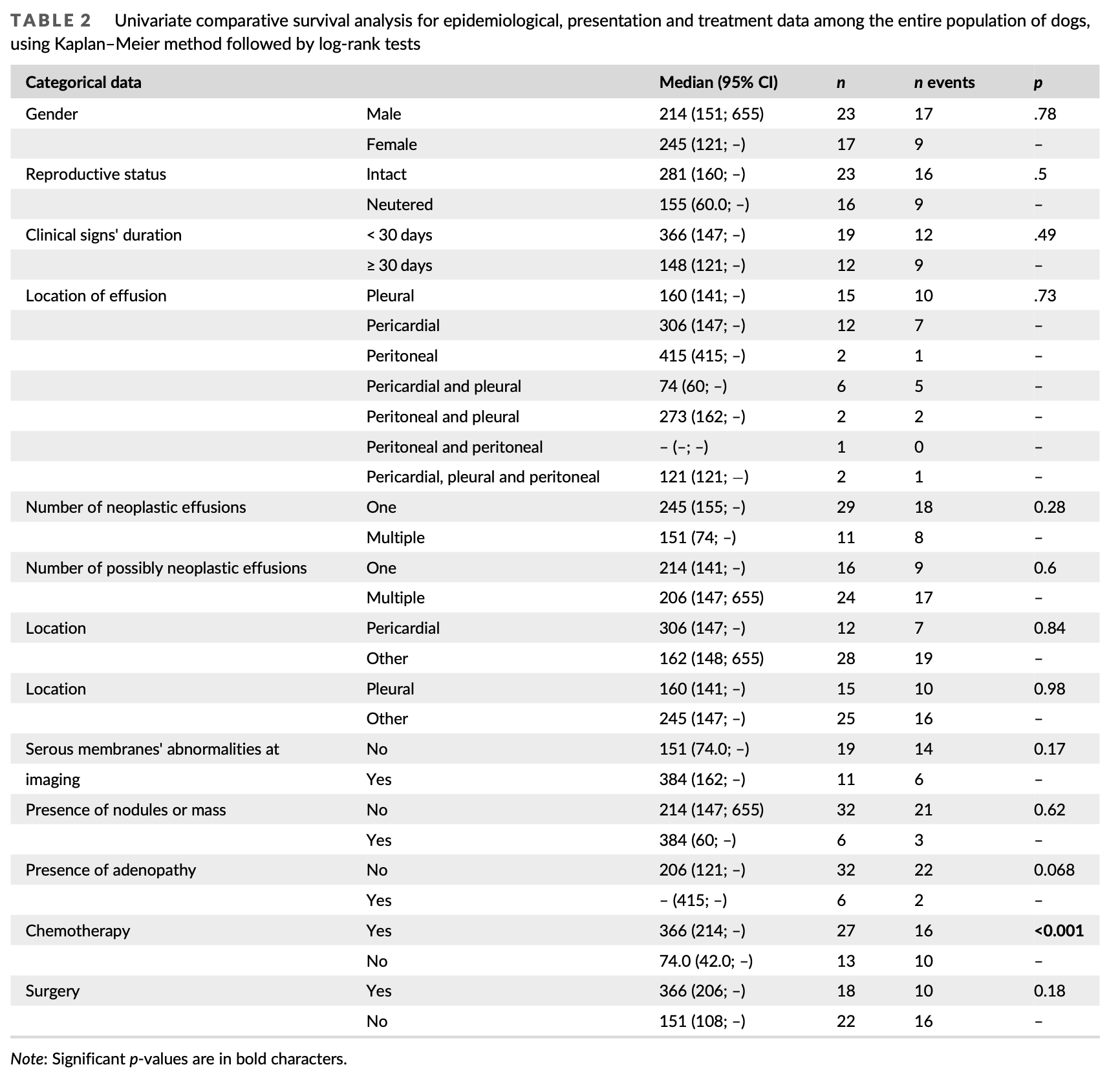
Forty dogs met the inclusion criteria which are fully displayed Table S1. The median age was 10.2 years (range: 3–13 years). There were 23 males including 18 intact, 4 neutered, and 1 for whom the reproductive status was unknown. There were 17 females including 5 intact, and 12 spayed. Most dogs were purebred (36/40). Golden Retriever was the most represented breed (11/40). Other breeds rep- resented by more than one dog included German Shepherd (4/40), Yorkshire Terrier (4/40), Bernese Mountain Dog (3/40) and American Staffordshire Terrier (2/40).
The median duration of clinical signs before the first morphologi- cal diagnosis was 14 days (range 1–395 days, n = 31). Mesothelioma was pleural in 15 dogs, pericardial in 12, peritoneal in 2, pericardial and pleural in 6, peritoneal and pleural in 2, peritoneal and pericardial in 1 and pericardial, pleural, and peritoneal in 2. At presentation, effu- sions concerned several body cavities in 26 dogs (65%). Among them, 10 presented confirmed multiple neoplastic effusions, 4 presented only one tumoral effusion, and 12 presented one confirmed neoplastic effusion and other effusions of unknown nature. Fourteen dogs pres- ented a unique tumoral effusion at presentation; among them, 2 had a
history of other effusions. For statistical analyses, effusions of unknown nature suspected of being mesothelioma-related were noted as suspectedly neoplastic.
3.2 | Imaging procedures
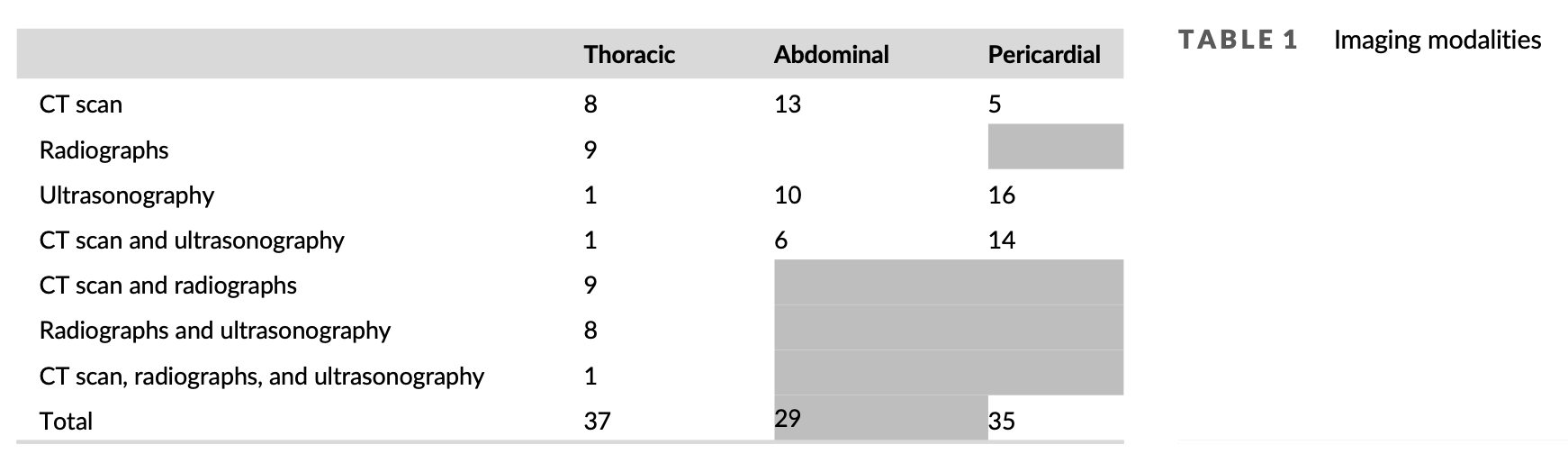
Pleural cavity imaging was performed in 37 dogs (92.5%) with computed tomography (CT) scan (19), radiographs (27) or, ultrasonography (11). Pericardial cavity imaging was performed in 35 dogs (87.5%) with CT scan (19) or, echocardiography (30). Abdominal imaging was performed in 29 dogs (72.5%) with CT scan (19) or, ultrasonography (16) (Table 1).
Imaging reports were retrospectively reviewed in 38 dogs; serous membranes were within normal limits (21/38, 55.3%), thickened (11/38, 28.9%), presented nodules (4/38, 10.5%), presented a mass (2/38, 5.3%), or were focally enhanced without hypertrophy at CT scan (2/38, 5.3%). Two dogs had both nodules and thickening of serous membranes. Both dogs presenting a mass had a histopathologi- cal diagnosis confirming mesothelial proliferation; one mass was heart-based, multilobulated and heterogeneous and the other was located at the right atrium, irregularly shaped, heterogenous, and poorly defined. Four dogs presented very mild intracavitary lymphade- nopathies and two dogs presented moderate sus-sternal lymphade- nopathy, though the lesions could not be aspirated due to their small size. One dog presented a lung-lobe torsion along with pleural and pericardial effusion; mesothelioma was histologically diagnosed on the parietal aspect of the torn lobe after the dog underwent lobec- tomy and pericardiectomy.
3.3 | Morphological assessment
All dogs had a cytopathological evaluation of their effusions and it was suggestive of mesothelioma in 36/40 (90%). Three cytopathological examinations were unconclusive due to poor cellularity. For those three cases, subsequent histopathological examination was suggestive of mesothelioma. In the remaining case, cytopathological examination rev- ealed a proliferation of well-differentiated mesothelial cells without any criteria of malignancy; diagnosis of mesothelioma was supported by his- topathology and immunohistochemistry.
Histopathology was performed in 19 dogs and was evocative of mesothelioma in 16/19 (84.2%). In two dogs, histopathology suggested a mesothelial hyperplasia of unknown origin and for one dog, biopsies were of poor quality. All three had pericardial effusion for which the cytopathological evaluation was consistent with meso- thelioma. All underwent pericardiectomy and the diagnosis was supported after recollection of the effusion in the pleural space and confirmed by a second cytopathological examination. On the 16 speci- mens with a tumoral diagnosis at histopathology, six presented emboli (37.5%); they were lymphatic in four, lymphatic and vascular in one, and not otherwise specified in one.
Immunohistostaining was performed in 10 dogs with: AE1/AE3 and vimentin (6/10), AE1/AE3 and smooth actin (1/10), AE1/AE3
alone (2/10) and vimentin alone (1/10). One dog presented positivity for AE3 and smooth actin, and negativity for AE1; results were all pos- itive for every other cases. Immunocytostaining was performed in 3 dogs with AE1/AE3 and vimentin, and all were double positive.
3.4 | Treatment
Twenty seven dogs received chemotherapy (group 1) and 13 did not (group 2). Chemotherapy was administered intracavitary in 12 dogs (44.4%), intravenously in 2 (7.4%) and both intracavitary and intrave- nously in 13 (48.1%). Agents used for intracavitary chemotherapy included cisplatin (13/25, 52%) and carboplatin (12/25, 48%). Agents used for intravenous chemotherapy included carboplatin (5/15, 33.3%), doxorubicin (4/15, 26.7%), alternance of carboplatin and doxorubicin (4/15, 26.7%), alternance of carboplatine and mitoxantrone (1/15, 6.6%) and a combination of cisplatin, carboplatin, mitomycin C and mitoxantrone (1/15, 6.6%). Eleven dogs received several molecules as part of their initial chemotherapy protocol (41%). One dog received metronomic therapy with chlorambucil at the end of his protocol. Response to chemotherapy was reported in 26 dogs and was complete 3 weeks after the first administration in 13 (50%). Follow-up of nodules was available in 2 dogs; one was reassessed after 1 month and presented disappearance of all nodules and the other one was reassessed after 5 months and presented nodules size’s reduction. Dogs with uncomplete response after 3 weeks of treatment did not experience CR afterwards. Number of administrations varied from 1 to 10 (median = 4; n = 23). Four adverse events were recorded: grade 3 and grade 4 neutropenia, grade 2 thrombocytopenia, and grade 2 vomiting. No renal toxicity was reported.
Ten complete responders experienced a relapse after a median PFI of 242.5 days. Among all dogs undergoing chemotherapy, nine dogs received a second protocol after relapse (4/27, 14.8%) or follow- ing absence of response to the first-line treatment (5/27, 18.5%). These included repeating previous chemotherapy protocol (2/27, 7.4%), initiation of another maximum tolerated dose protocol (5/27, 18.5%), administration of toceranib (2/27, 7.4%), and pleurodesis (2/27, 7.4%). Three out of 4 dogs who had a CR to their first protocol experienced a CR to the second protocol; one was treated with toceranib, and two received a repeatition of their first platinum-based
intracavitary protocol. Dogs not achieving a CR with their first proto- col did not achieve a better response after the rescue protocol.
Eighteen dogs (45%) underwent therapeutic surgical procedures consisting of pericardiectomy (13/18, 72.2%), lobectomy and peri- cardiectomy (1/18, 5.6%), pleural port placement (3/18, 16.7%), and pleural port placement and pericardiectomy (1/18, 5.6%). Among them, only two dogs were from group 2; both underwent pericardiectomy.
Other treatments administered included prednisolone (27/40, 67.5%), non-steroidal anti-inflammatory drugs (6/40, 15%), furose- mide (8/40, 20%) and sotalol (1/40, 2.5%). Seven dogs did not receive complementary treatments—4 from group 1 and 3 from group 2.
3.5 | Survival analysis and prognostic factors
Twenty-six dogs died of tumour-related cause and 14 dogs were cen- sored. Six died of other causes (cardiac arrest during surgery after relapse, piroplasmosis, leishmaniasis, histiocytic sarcoma and hit by car) and one died in unknown circumstances while his mesothelioma was still in remission. Six dogs were lost to follow-up after a median follow-up time of 273 days (range 41–779 days), and one was still alive at the time of data collection (after 274 days of follow-up).
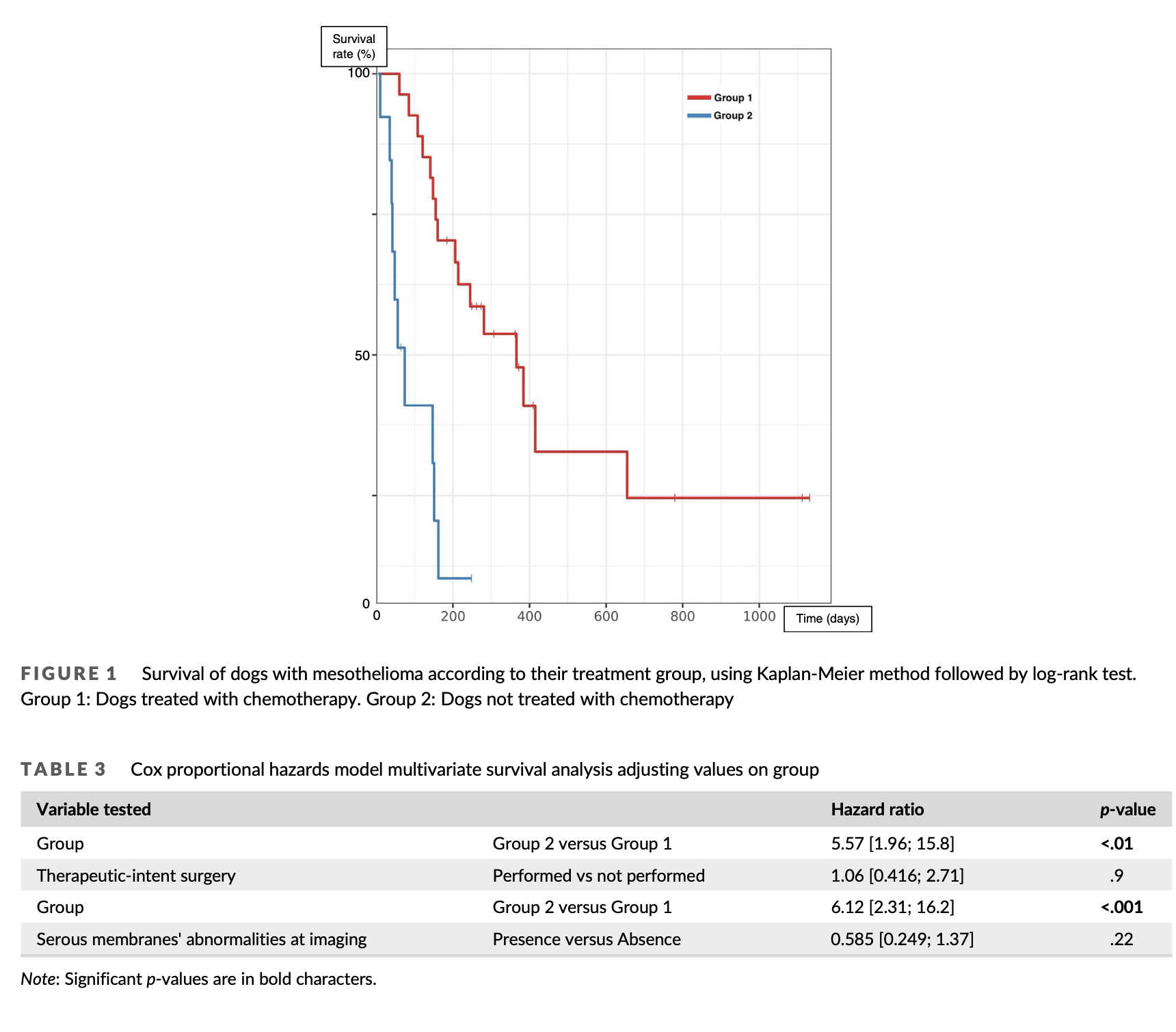
Group 1 and 2 were homogeneous except for performance of therapeutic-intent surgery: there was a significantly higher percentage of dogs undergoing surgery in group 1 than group 2 (p = .016). Among all factors tested for their impact on survival on univariate analysis, only chemotherapy was significantly associated with outcome, with a median survival time (MST) of 366 days for dogs from group 1 and 74 days for dogs from group 2 (p < .001) (Table 2, Figure 1). Dogs under- going surgery as part of their treatment protocol tended to live longer than others, but this was not statistically significant (MST 366 vs. 151 days, p = .18). Dogs with serous membranes’ abnormalities at imaging also tended to live longer (MST 288 vs. 155 days, p = .17). A multivari- ate analysis including all three parameters could not be conducted due to the low number of cases; therefore, two distinct multivariate analyses were conducted using chemotherapy performance as the first variable, and imaging results or surgery performance as the sec- ond. In both analyses, only chemotherapy remained significantly asso- ciated with survival (hazard ratio 5.37–6.12; p < .01, Table 3).
Among group 1, response to treatment was the sole prognostic factor found in univariate analysis; complete responders after the first administration of chemotherapy had a MST of 415 days compared to 160 days for uncomplete responders (p < .01) (Table 4, Figure 2). Mul- tivariate analysis could not be performed due to the limited number of cases.
3.6 | Survival analysis among subpopulations
Considering only pleural mesothelioma, chemotherapy was the only treatment significantly associated with outcome (MST 214 vs. 40 days, p < .001). Surgery was not significantly associated with sur- vival for pleural mesothelioma (MST 281 vs. 150 days, p = .38).
Considering only pericardial mesothelioma, dogs treated with che- motherapy (MST 366 vs. 102 days, p = .086) and/or surgery (MST 366 vs. 147 days, p = .42) tended to live longer but this was not sig- nificant. Regarding dogs with multicavitary effusions, chemotherapy was positively associated with outcome (MST 366 vs. 74days, p > .001) and surgery was not significantly associated with outcome (MST 366 vs. 147 days, p = .15). Multivariate studies could not be conducted due to the low number of cases. Full results are displayed Table S2.
Among dogs having a tumoral diagnosis at histopathology, all dogs presenting emboli (n = 6) were treated with chemotherapy and 5/6 had surgery. Among them, 3/6 had CR to chemotherapy. Dogs without emboli had a MST of 655 days compared to 310 for those who did, though this was not significant (p = .43) (Table S3).
4 | DISCUSSION
Apart from a recently published retrospective study describing a rela- tively large series of mesothelioma,16 data on this tumour is rather scattered in the veterinary literature. To discuss our results, a system- atic review was conducted. This review does not include the results of the study of Moberg et al. which are discussed separately. A total number of 122 cases of mesothelioma, fulfilling the four inclusion criteria set in our study, were found complicated in 47 isolated case reports and 10 case series.1,6,13,17,18,22–73 Morphological evaluation was made by repeated cytopathological examinations in one case and histopathology in every other cases. Forty-four had immunohisto- chemistry performed. In the study of Moberg et al., 34 cases diag- nosed with histopathology (32/34) or cell-block cytopathology (2/34) were described. Seventeen had immunohistochemistry performed.
In our population, pleural (62.5%) and pericardial (50%) cavities were the most commonly affected. Similarly, in Moberg’s series, a majority of dogs had involvement of the pleural cavity (91.2%) and pericardial cavity was the second most commonly affected (47%).16 In
our literature review, location was known in 120 dogs; it was pleural in 13 (10.8%), pericardial in 77 (64.2%), peritoneal in 15 (12.5%), peri- cardial and pleural in 6 (5%), pleural and peritoneal in 3 (2.5%), perito- neal and pericardial in 1 (0.8%%), tri-cavitary in 2 (1.7%) and concerned the tunica vaginalis in 3 (2.5%). Thus, pleural cavity was involved in 24% and pericardial cavity in 71.7% of cases. This differ- ence could result from a selection bias; in fact, 61 pericardial meso- theliomas were included from case series on pericardial disease or pericardiectomy.1,6,38,39,45,57 In contrast, no mesothelioma was included from cohorts of pleural or peritoneal diseases. Finally, as in humans, canine mesothelioma seems to mainly concern pleural cavity; though, pericardial cavity may be more frequently involved in dogs.11,74
Medical imaging of the neoplastic cavity is required during the diagnosis of mesothelioma, especially to exclude other cause of effu- sion. Indeed, little is known about mesothelioma imaging; two studies on thoracic CT images of malignant and benign diseases showed pari- etal pleura thickening, nodular diaphragmatic thickening and thoracic wall invasion to be associated with thoracic malignancies.75,76 Though, 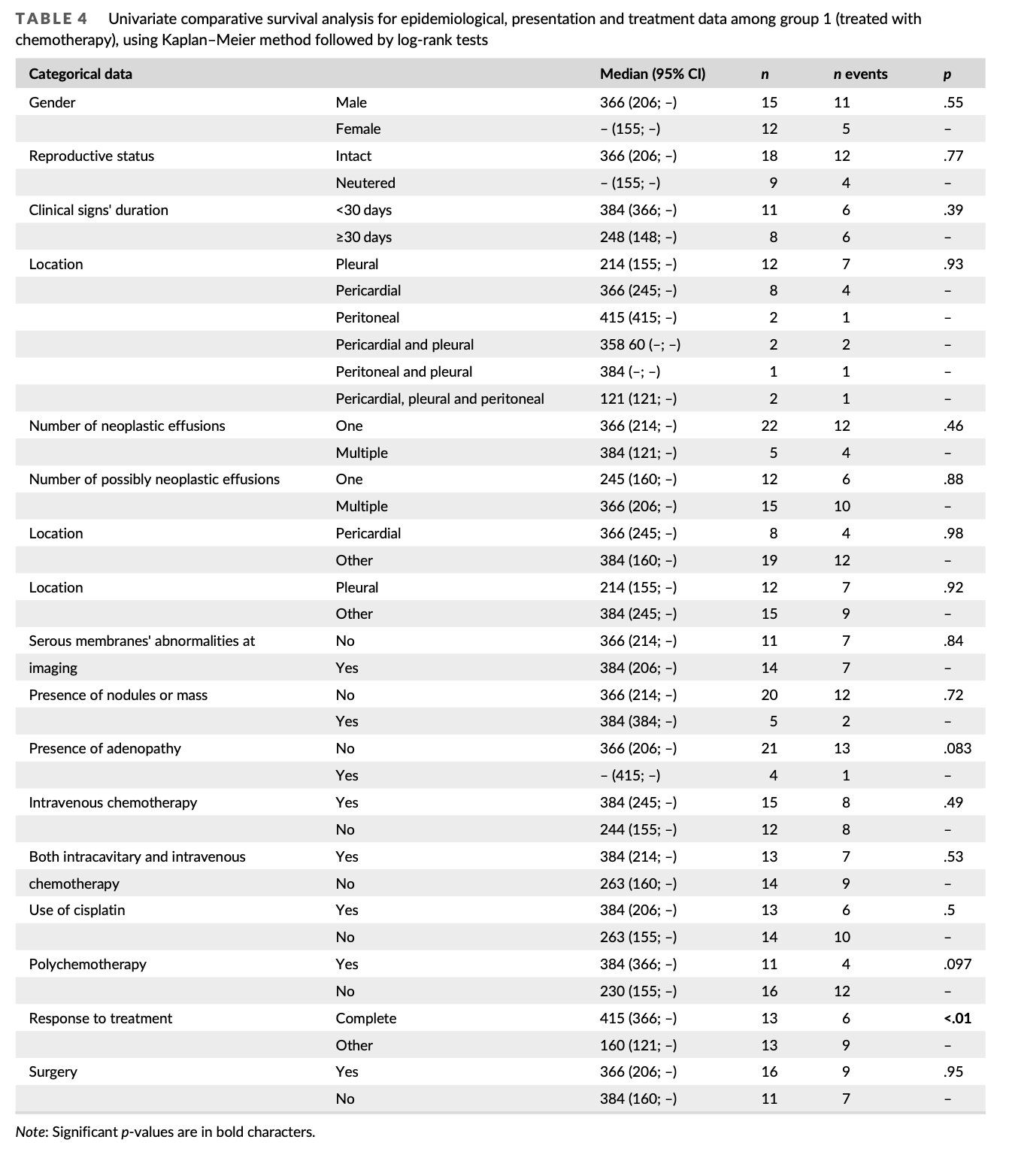
all intra-thoracic malignancies were treated at the same level and only a limited number of mesothelioma were included. In our cohort, medi- cal imaging was unremarkable in 55.3% of cases; when abnormal, main finding was thickening of serous membrane (28.9%), and less fre- quently nodules or masses (15.8%). In Moberg’s series, 27 description of thoracic and abdominal CT were available. No gross diseas was
identified in 33% of dogs; nodules were quite common (≥33%) as well as pleural (30%) and/or pericardial (11%) thickening. In previously published mesothelioma cases, imaging procedures’ information were available for 56 dogs; thoracic/pericardial cavity was evaluated in 47 and abdominal cavity in 21. No abnormal lesion was found in 30 dogs (53.6%); common findings were serosal nodules/masses
(17/56, 30.4%) and serosal thickening (9/56, 16.1%). Results should be compared with caution since they were obtained with several imaging techniques over a long period of time. However, results show that absence of imaging abnormalities is common. When present, serosal thickening, nodules and masses are the most common abnormalities.
In our series, chemotherapy was the only treatment significantly
and independently associated with survival. MST was 366 days for
dogs receiving chemotherapy compared to 74 days for those who did
not. Moberg found similar results in a cohort of 34 dogs, with a MST
from diagnosis to death of 234 days for 25 dogs treated with chemo-
therapy and 29days for others.16 Twenty-four other cases of dogs
receiving chemotherapy for the treatment of mesothelioma were identified in literature.1,17,18,30,32,33,36,39–41,46,47,49,53,56,61,69,70,73 It
was administered intracavitary in 14 (58.3%), intravenously in 7 (29.1%) and both in 3 (12.5%). Intracavitary molecules administered were cisplatin (10), carboplatin (4), mitoxantrone and carboplatin (1), gemcitabine and carboplatin (1), and paclitaxel-loaded adipose tissue (1). Intravenous molecules were doxorubicin (5), mitoxantrone (2), cis- platin (1) and carboplatin (1). In published cases, dogs treated with chemotherapy also tented to survive longer (Table S4), even though there could be a selection bias. In the end, the three cohorts seem to indicate a benefit of chemotherapy.
In our study, response to chemotherapy was the only prognostic factor associated with survival among dogs treated with chemother- apy. After the first chemotherapy session, 50% were in CR. Moberg et al. reported 4% of complete responders and 37% of responders
when assessing the response clinically and by quantification of resid- ual effusion drainage, 3 weeks after the initiation of chemotherapy. Response to chemotherapy was assessed in 13 other published cases according to the criteria defined in our study; it was complete in 3/13 dogs. Differences in complete response rates must be interpreted cau- tiously considering possible evaluation bias and the rather small num- ber of cases per group. Though, it could also be linked to the different protocols performed. For instance, cisplatin was used in 52% of dogs in our study, 46% in our literature review and no dog in Moberg’s study. In humans, cisplatin is part of the first-line treatment of meso- thelioma.10–12,77 In dogs, it is not frequently used, mainly because of its associated nephrotoxicity; though, it seems well tolerated when administered intracavitary with adequate saline diuresis.17,78 No case of nephrotoxicity was reported in our cohort. Moreover, several mole- cules were administered as the first-line chemotherapy in 41% of dogs in our cohort, 25% in our literature review and 17% in Moberg’s cohort. In humans, response rates are higher with multi-agent proto- cols compared to single-agent protocols.79
In our study, dogs treated with surgery tended to live longer even though it was not significant. In Moberg’s series, 20 dogs underwent perciardectomy and 22 dogs had a subcutaneous pleural and/or peri- toneal port placed but outcome associated with those procedures was not displayed.16 In literature, surgery was performed in 71/105 (67.6%) dogs. It consisted of pericardiectomy in 61 (85.9%), debulking in 9 (12.6%), and both in 1 (1.4%). Dogs treated with surgery tended to survive longer; but, as in our cohort, most dogs received either sur- gery and chemotherapy or no treatment. In our cohort, only two dogs
received surgery in the non-chemo group, which is not enough to assess effectiveness. When limiting our analysis to dogs receiving che- motherapy, no tendency could be found between surgery and out- come either in literature or in our cohort. Influence of surgery on outcome need to be further assessed; ideally, prospective studies on specific location with comparison of several type of surgical proce- dures would be necessary.
Main limitations of our study are due to its retrospective nature. Diagnostic, staging, and treatment protocols were not standardized. Multivariate analyses were necessary to conclude in most cases but could not always be performed due to the limited number of cases included. Even though complete staging was not performed in all dogs, imaging of the neoplastic cavity was always performed. There- fore, diagnosis based on cytopathological examinations were autho- rized, since cases of carcinomatous or sarcomatous effusions without any mass in the affected cavity appears unlikely.80 Furthermore, in human medicine, cytology can be an acceptable mean of diagnostic con- firmation when clinical and imaging context are in favour of mesotheli- oma and biopsies cannot be collected.10,81 Moreover, histopathological examinations are not always reliable and immunohistochemistry can only confirm the mesothelial origin and not the neoplastic nature of the proliferation.6 This is why morphological examination was always con- fronted to clinical and imaging results in our study, which decreases the risk of misdiagnosis.
5 | CONCLUSION
Conclusively, chemotherapy was associated with an increased survival of dogs with mesothelioma in our cohort; complete response after the first administration was the only identified positive prognostic factor. Impact of surgery on survival remains unknown and might depend of neoplastic location.
ACKNOWLEDGEMENTS
Authors would like to deeply thank Dr Esther Piccirillo and Dr Laurie Boissy for their help in collecting a part of the files. Authors thank the veterinary hospital Advetia (Velizy-Villacoublay, France) and the veter- inary school of Toulouse, from where some cases were included. Authors also thank Dr Isabelle Bublot (former practitioner in cardiol- ogy at VetAgro Sup) and the intensive care unit of VetAgro Sup for their help in the management of some of the cases and their help in the preliminary work in this study. Finally, authors thank all referring veterinarians, radiologists, cytopathologists and pathologists who have been involved in the diagnosis of the cases.
FUNDING INFORMATION
This work received no funding.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ORCID
David Sayag
https://orcid.org/0000-0001-5084-8892
REFERENCES
1. Stepien RL, Whitley NT, Dubielzig RR. Idiopathic or mesothelioma- related pericardial effusion: clinical findings and survival in 17 dogs studied retrospectively. J Small Anim Pract. 2000;41(8):342-347. doi:10.1111/j.1748-5827.2000.tb03215.x
2. Cagle LA, Epstein SE, Owens SD, Mellema MS, Hopper K, Burton AG. Diagnostic yield of cytologic analysis of pericardial effusion in dogs. J Vet Intern Med. 2014;28(1):66-71. doi:10.1111/jvim.12253
3. Munday JS, Löhr CV, Kiupel M. Tumors of the alimentary tract. Tumors in Domestic Animals. John Wiley & Sons, Ltd; 2016:499-601. doi:10.1002/9781119181200.ch13
4. Goupil A, Bolliger C, Lapointe C, Morency A, Girard C. Embolised mesothelial cells in a tracheobronchial lymph node associated with idiopathic chylopericardium in a dog. J Small Anim Pract. 2012;53(11): 664-667. doi:10.1111/j.1748-5827.2012.01285.x
5. Peters M, Tenhündfeld J, Stephan I, Hewicker-Trautwein M. Embolized mesothelial cells within mediastinal lymph nodes of three dogs with idiopathic haemorrhagic pericardial effusion. J Comp Pathol. 2003;128(2–3):107-112. doi:10.1053/jcpa.2002.0612
6. Milne E, Martinez Pereira Y, Muir C, et al. Immunohistochemical dif- ferentiation of reactive from malignant mesothelium as a diagnostic aid in canine pericardial disease: immunohistochemistry of canine mesothelioma. J Small Anim Pract. 2018;59(5):261-271. doi:10.1111/ jsap.12830
7. Tickman RJ, Cohen C, Varma VA, Fekete PS, DeRose PB. Distinction between carcinoma cells and mesothelial cells in serous effusions use- fulness of Immunohistochemistry. Acta Cytol. 1990;34(4):491-496.
8. Andreasen CB, Mahaffey EA, Duncan JR. Intermediate filament staining in the cytologic and histologic diagnosis of canine skin and soft tissue tumors. Vet Pathol. 1988;25(5):343-349. doi:10.1177/ 030098588802500502
9. Desnoyers MM, Haines DM, Searcy GP. Immunohistochemical detec- tion of intermediate filament proteins in formalin fixed normal and neoplastic canine tissues. Can J Vet Res Rev Can Rech Veterinaire. 1990;54(3):360-365.
10. Janes SM, Alrifai D, Fennell DA. Perspectives on the treatment of malignant pleural mesothelioma. N Engl J Med. 2021;385(13):1207- 1218. doi:10.1056/NEJMra1912719
11. Nadal E, Bosch-Barrera J, Cedrés S, et al. SEOM clinical guidelines for the treatment of malignant pleural mesothelioma (2020). Clin Transl Oncol. 2021;23(5):980-987. doi:10.1007/s12094-020-02532-2
12. Board PATE. Malignant Mesothelioma Treatment (Adult) (PDQ®). National Cancer Institute, 2021. Accessed August 9, 2021. https:// www.ncbi.nlm.nih.gov/books/NBK65983/
13. Atencia S, Doyle RS, Whitley NT. Thoracoscopic pericardial window for management of pericardial effusion in 15 dogs. J Small Anim Pract. 2013;54(11):564-569. doi:10.1111/jsap.12138
14. Jackson J, Richter KP, Launer DP. Thoracoscopic partial Peri- cardiectomy in 13 dogs. J Vet Intern Med. 1999;13(6):529-533. doi: 10.1111/j.1939-1676.1999.tb02205.x
15. Michelotti KP, Youk A, Payne JT, Anderson J. Outcomes of dogs with recurrent idiopathic pericardial effusion treated with a 3-port right- sided thoracoscopic subtotal pericardiectomy. Vet Surg. 2019;48(6): 1032-1041. doi:10.1111/vsu.13223
- Moberg HL, Gramer I, Schofield I, et al. Clinical presentation, treat- ment and outcome of canine malignant mesothelioma: a retrospective study of 34 cases. Vet Comp Oncologia. 2021;20:1-9. doi:10.1111/ vco.12777
- Moore AS, Kirk C, Cardona A. Intracavitary cisplatin chemotherapy experience with six dogs. J Vet Intern Med. 1991;5(4):227-231. doi: 10.1111/j.1939-1676.1991.tb00953.x
- Spugnini EP, Crispi S, Scarabello A, Caruso G, Citro G, Baldi A. Piroxicam and intracavitary platinum-based chemotherapy for the treatment of advanced mesothelioma in pets: preliminary observations. J Exp Clin Cancer Res CR. 2008;27(1):6. doi:10.1186/1756-9966-27-6
- LeBlanc AK, Atherton M, Bentley RT, et al. Veterinary cooperative oncology group-common terminology criteria for adverse events (VCOG-CTCAE v2) following investigational therapy in dogs and cats.Vet Comp Oncol. 2021;19(2):311-352. doi:10.1111/vco.12677
- Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a veterinary cooperative oncology group (VCOG) consensus document. Vet Comp Oncologia. 2015;13(3):176-183. doi:10.1111/vco.12032
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi:10.1016/j.ejca.2008.10.026
- Breeze R, Lauder I. Pleural mesothelioma in a dog. Vet Rec. 1975; 96(11):243-246. doi:10.1136/vr.96.11.243
- van Ooijen PG. Exfoliative cytology in the diagnosis of diffuse meso- thelioma in the dog. Illustration by a case report. Tijdschr Diergeneeskd. 1978;103(20):1116-1120.
- Ikede BO, Zubaidy A, Gill CW. Pericardial mesothelioma with cardiac tamponade in a dog. Vet Pathol. 1980;17(4):496-500. doi:10.1177/ 030098588001700412
- Trigo FJ, Morrison WB, Breeze RG. An ultrastructural study of canine mesothelioma. J Comp Pathol. 1981;91(4):531-537. doi:10.1016/ 0021-9975(81)90081-5
- Harbison ML, Godleski JJ. Malignant mesothelioma in urban dogs. Vet Pathol. 1983;20(5):531-540. doi:10.1177/030098588302000504
- Craig JA, Helman RG, Walker M. Costal bone changes similar to
hypertrophic osteopathy associated with pulmonary and abdominal mesothelioma in a dog. J Am Vet Med Assoc. 1985;186(10):1100- 1101.
- Cihak RW, Roen DR, Klaassen J. Malignant mesothelioma of the tunica vaginalis in a dog. J Comp Pathol. 1986;96(4):459-462. doi:10. 1016/0021-9975(86)90041-1
- Smith DA, Hill FWG. Metastatic malignant mesothelioma in a dog. J Comp Pathol. 1989;100(1):97-101. doi:10.1016/0021-9975(89) 90094-7
- Ogilvie GK, Reynolds HA, Richardson RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. 1989;195(11):1580-1583.
- Forbes DC, Matthews BR. Abdominal mesothelioma in a dog. Can Vet J. 1991;32(3):176-177.
- Ogilvie GK, Obradovich JE, Elmslie RE, et al. Efficacy of mitoxantrone against various neoplasms in dogs. J Am Vet Med Assoc. 1991;198(9): 1618-1621.
- Keller ET, Vail DM. Intrapleural administration of cisplatin (DDP) for treatment of pleural neoplasia. J Vet Intern Med. 1992;6(3):198-199.
- Leisewitz AL, Nesbit JW. Malignant mesothelioma in a seven-week- old puppy. J S Afr Vet Assoc. 1992;63(2):70-73.
- Schoning P, Layton CE, Fortney WD, Willard LH, Cook JE. Sclerosing peritoneal mesothelioma in a dog evaluated by electron microscopy and immunoperoxidase techniques. J Vet Diagn Invest. 1992;4(2):217- 220. doi:10.1177/104063879200400226
- McDonough SP, MacLachlan NJ, Tobias AH. Canine pericardial meso- thelioma. Vet Pathol. 1992;29(3):256-260. doi:10.1177/ 030098589202900312
37. DiPinto M, Dunstan R, Lee C. Cystic, peritoneal mesothelioma in a dog. J Am Anim Hosp Assoc. 1995;31(5):385-389. doi:10.5326/ 15473317-31-5-385
38. Kerstetter KK, Krahwinkel DJ, Millis DL, Hahn K. Pericardiectomy in dogs: 22 cases (1978-1994). J Am Vet Med Assoc. 1997;211(6):736-740. 39. Dunning D, Monnet E, Orton EC, Salman MD. Analysis of prognostic indicators for dogs with pericardial effusion: 46 cases (1985-1996).
J Am Vet Med Assoc. 1998;212(8):1276-1280.
40. Closa JM, Font A, Mascort J. Pericardial mesothelioma in a dog: long-
term survival after pericardiectomy in combination with chemother- apy. J Small Anim Pract. 1999;40(8):383-386. doi:10.1111/j.1748- 5827.1999.tb03105.x
41. Neath PJ, Brockman DJ, King LG. Lung lobe torsion in dogs: 22 cases (1981-1999). J Am Vet Med Assoc. 2000;217(7):1041-1044. doi:10. 2460/javma.2000.217.1041
42. Dias Pereira P, Azevedo M, Gärtner F. Case of malignant biphasic mesothelioma in a dog. Vet Rec. 2001;149(22):680-681. doi:10.1136/ vr.149.22.680
43. Kim JH, Choi YK, Yoon HY, Kweon OK, Kim DY. Juvenile malignant mesothelioma in a dog. J Vet Med Sci. 2002;64(3):269-271. doi:10. 1292/jvms.64.269
44. Geninet C, Bernex F, Rakotovao F, Crespeau FL, Parodi AL, Fontaine JJ. Sclerosing peritoneal mesothelioma in a dog – a case report. J Vet Med Ser A. 2003;50(8):402-405. doi:10.1046/j.0931- 184X.2003.00566.x
45. Machida N, Tanaka R, Takemura N, Fujii Y, Ueno A, Mitsumori K. Development of pericardial mesothelioma in golden retrievers with a long-term history of idiopathic haemorrhagic pericardial effusion. J Comp Pathol. 2004;131(2–3):166-175. doi:10.1016/j.jcpa.2004. 03.002
46. Charney SC, Bergman PJ, McKnight JA, et al. Evaluation of intracavitary mitoxantrone and carboplatin for treatment of carcino- matosis, sarcomatosis and mesothelioma, with or without malignant effusions: a retrospective analysis of 12 cases (1997-2002)*. Vet Comp Oncol. 2005;3(4):171-181. doi:10.1111/j.1476-5810.2005. 00075.x
47. Reggeti F, Brisson B, Ruotsalo K, Southorn E, Bienzle D. Invasive epi- thelial mesothelioma in a dog. Vet Pathol. 2005;42(1):77-81. doi:10. 1354/vp.42-1-77
48. Sato T, Miyoshi T, Shibuya H, Fujikura J, Koie H, Miyazaki Y. Perito- neal biphasic mesothelioma in a dog. J Vet Med Ser A. 2005;52(1):22- 25. doi:10.1111/j.1439-0442.2004.00680.x
49. Brisson BA, Reggeti F, Bienzle D. Portal site metastasis of invasive mesothelioma after diagnostic thoracoscopy in a dog. J Am Vet Med Assoc. 2006;229(6):980-983. doi:10.2460/javma.229.6.980
50. Brower A, Herold LV, Kirby BM. Canine cardiac mesothelioma with granular cell morphology. Vet Pathol. 2006;43(3):384-387. doi:10. 1354/vp.43-3-384
51. Morini M, Bettini G, Morandi F, Burdisso R, Marcato P. Deciduoid peritoneal mesothelioma in a dog. Vet Pathol. 2006;43(2):198-201. doi:10.1354/vp.43-2-198
52. Liptak JM, Brebner NS. Hemidiaphragmatic reconstruction with a transversus abdominis muscle flap after resection of a solitary dia- phragmatic mesothelioma in a dog. J Am Vet Med Assoc. 2006;228(8): 5-1208.
53. Seo KW, Choi US, Jung YC, et al. Palliative intravenous cisplatin treat- ment for concurrent peritoneal and pleural mesothelioma in a dog. J Vet Med Sci. 2007;69(2):201-204. doi:10.1292/jvms.69.201
54. Echandi RL, Morandi F, Newman SJ, Holford A. Imaging diagnosis— canine thoracic mesothelioma. Vet Radiol Ultrasound. 2007;48(3):243- 245. doi:10.1111/j.1740-8261.2007.00236.x
55. Vural SA, Ozyildiz Z, Ozsoy SY. Pleural mesothelioma in a nine- month-old dog. Iran Vet J. 2007;60(1):30-33. doi:10.1186/2046- 0481-60-1-30
- Avakian A, Alroy J, Rozanski E, Keating J, Rosenberg A. Lipid-rich pleural mesothelioma in a dog. J Vet Diagn Invest. 2008;20(5):665- 667. doi:10.1177/104063870802000525
- MacDonald KA, Cagney O, Magne ML. Echocardiographic and clinico- pathologic characterization of pericardial effusion in dogs: 107 cases (1985-2006). J Am Vet Med Assoc. 2009;235(12):1456-1461. doi:10. 2460/javma.235.12.1456
- Espino L, Vazquez S, Faílde D, Barreiro A, Miño N, Goicoa A. Local- ized pleural mesothelioma causing cranial vena cava syndrome in a dog. J Vet Diagn Invest. 2010;22(2):309-312. doi:10.1177/ 104063871002200228
- Gumber S, Fowlkes N, Cho DY. Disseminated sclerosing peritoneal mesothelioma in a dog. J Vet Diagn Invest. 2011;23(5):1046-1050. doi:10.1177/1040638711416625
- Vascellari M, Carminato A, Camali G, Melchiotti E, Mutinelli F. Malignant mesothelioma of the tunica vaginalis testis in a dog: histo- logical and Immunohistochemical characterization. J Vet Diagn Invest. 2011;23(1):135-139. doi:10.1177/104063871102300125
- Gallach RG, Mai W. Cardiac MRI findings in a dog with a diffuse peri- cardial mesothelioma and pericardial effusion. J Am Anim Hosp Assoc. 2013;49(6):398-402. doi:10.5326/JAAHA-MS-5925
- Yamamoto S, Fukushima R, Kobayashi M, Machida N. Mixed form of pericardial mesothelioma with osseous differentiation in a dog. J Comp Pathol. 2013;149(2–3):229-232. doi:10.1016/j.jcpa.2013.01.009
- de Brot S, Hilbe M. Pulmonary alveolar microlithiasis with concurrent pleural mesothelioma in a dog. J Vet Diagn Invest. 2013;25(6):798- 802. doi:10.1177/1040638713504571
- D’Angelo AR, Di Francesco G, Rosaria G. Sclerosing peritoneal meso- thelioma in a dog: histopathological, histochemical and immunohisto- chemical investigations. Vet Ital. 2014;50(4):301-305. doi:10.12834/ VetIt.20.1309.130
- Stevens BJ, Montgomery SA, Phillips KL, Wester MW, Jennings SH. Pathology in practice. J Am Vet Med Assoc. 2014;245(1):57-59. doi: 10.2460/javma.245.1.57
- Di Tommaso M, Rocconi F, Marruchella G, et al. Invasive pleural malignant mesothelioma with rib destruction and concurrent osteo- sarcoma in a dog. Acta Vet Scand. 2015;57:85. doi:10.1186/s13028- 015-0176-1
- Son NV, Chambers JK, Shiga T, et al. Sarcomatoid mesothelioma of tunica vaginalis testis in the right scrotum of a dog. J Vet Med Sci. 2018;80(7):1125-1128. doi:10.1292/jvms.18-0186
- Pascotto E, Gianoncelli A, Calligaro C, et al. Ferruginous bodies resolved by synchrotron XRF in a dog with peritoneal malignant mesothelioma. Environ Sci Pollut Res Int. 2018;25(35):35707-35714. doi:10.1007/s11356-018-3521-x
- Nabeta R, Nakagawa Y, Chiba S, et al. Pericardial mesothelioma in a dog: the feasibility of ultrasonography in monitoring tumor progres- sion. Front Vet Sci. 2019;6:121. doi:10.3389/fvets.2019.00121
- Hartmann HF, De Oliveira MT, Feranti JPS, et al. Thoracoscopic peri- cardiectomy associated with fully implantable catheter via thoracoscopy in the management of mesothelioma in a bitch. J Vet Med Sci. 2019;81(6):946-948. doi:10.1292/jvms.17-0631
- Morgan KRS, Dominic CG, Beeler-Marfisi J, et al. Presumptive seeding metastasis of pericardial mesothelioma following repeated
pericardiocentesis in a dog. Can Vet J Rev Veterinaire Can. 2019;60(9):
972-975.
72. Rivera PA, Borgarelli M. Cardiovascular images: constrictive pericardi-
tis and tricavitary effusion in a dog with pericardial mesothelioma.
J Vet Cardiol. 2020;32:55-59. doi:10.1016/j.jvc.2020.09.005
73. Zeira O, Ghezzi E, Pettinari L, et al. Case report: microfragmented adi- pose tissue drug delivery in canine mesothelioma: a case report on safety, feasibility, and clinical findings. Front Vet Sci. 2020;7:585427.
doi:10.3389/fvets.2020.585427
74. McGehee E, Gerber DE, Reisch J, Dowell JE. Treatment and out-
comes of primary pericardial mesothelioma: a contemporary review of 103 published cases. Clin Lung Cancer. 2019;20(2):e152-e157. doi: 10.1016/j.cllc.2018.11.008
75. Watton TC, Lara-Garcia A, Lamb CR. Can malignant and inflammatory pleural effusions in dogs be distinguished using computed tomogra- phy? Vet Radiol Ultrasound off J Am Coll Vet Radiol Int Vet Radiol Assoc. 2017;58(5):535-541. doi:10.1111/vru.12534
76. Reetz JA, Suran JN, Zwingenberger AL, Stefanovski D. Nodules and masses are associated with malignant pleural effusion in dogs and cats but many other intrathoracic CT features are poor predictors of the effusion type. Vet Radiol Ultrasound off J Am Coll Vet Radiol Int Vet Radiol Assoc. 2019;60(3):289-299. doi:10.1111/vru.12706
77. Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: malignant pleural mesothelioma, version 3.2016. J Natl Compr Cancer Netw JNCCN. 2016;14(7):825-836. doi:10.6004/jnccn.2016.0087
78. Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 2008;6(1):1-18. doi:10. 1111/j.1476-5829.2007.00142.x
79. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol off J Am Soc Clin Oncol. 2003;21(14):2636-2644. doi:10.1200/JCO.2003.11.136
80. Weston PJ, Baines SJ, Finotello R, Mortier JR. Clinical, CT, and ultrasonographic features of canine and feline pleural and peritoneal carcinomatosis and sarcomatosis. Vet Radiol Ultrasound. 2021;62(3): 331-341. doi:10.1111/vru.12951
81. Alì G, Bruno R, Fontanini G. The pathological and molecular diagnosis of malignant pleural mesothelioma: a literature review. J Thorac Dis. 2018;10(Suppl 2):S276-S284. doi:10.21037/jtd.2017.10.125
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
How to cite this article: Lajoinie M, Chavalle T, Floch F, et al. Outcome of dogs treated with chemotherapy for mesothelioma: A retrospective clinical study on 40 cases and a literature review. Vet Comp Oncol. 2022;20(4):825‐835. doi:10.1111/vco.12843