Cancer is the most common cause of death in adult dogs. Many features of spontaneously developing tumors in pet dogs contribute to their potential utility as a human disease model. These include similar environmental exposures, similar clonal evolution as it applies to important factors such as immune avoidance, a favorable body size for imaging and serial biopsy, and a relatively contracted time course of disease progression, which makes evaluation of temporal endpoints such as progression free or overall survival feasible in a comparatively short time frame. These criteria have been leveraged to evaluate novel local therapies, demonstrate proof of tumor target inhibition or tumor localization, evaluate potential antimetastatic approaches, and assess the efficacy, safety and immune effects of a variety of immune-based therapeutics. Some of these canine proof of concept studies have been instrumental in informing subsequent human clinical trials. This review will cover key aspects of clinical trials in dogs with spontaneous neoplasia, with examples of how these studies have contributed to human cancer therapeutic development.
Keywords: dog, tumor, clinical trial, translational, animal model
Edited by:
Mark W. Dewhirst, Duke University, United States
Reviewed by:
Terence S. Herman, University of Oklahoma Health Sciences Center, United States Allison B. Warner, Memorial Sloan Kettering Cancer Center, United States Amy LeBlanc, National Cancer Institute (NCI), United States
*Correspondence:
Douglas H. Thamm dthamm@colostate.edu
Specialty section:
This article was submitted to Cancer Molecular Targets and Therapeutics, a section of the journal Frontiers in Oncology
Received: 31 July 2019 Accepted: 31 October 2019 Published: 15 November 2019
Citation:
Thamm DH (2019) Canine Cancer: Strategies in Experimental Therapeutics. Front. Oncol. 9:1257. doi: 10.3389/fonc.2019.01257
INTRODUCTION
Common concerns with regard to the clinical applicability of many murine models of human cancer include immune status, significantly reduced clonal heterogeneity, relative tumor burden, tumor location (orthotopic vs. heterotopic), species-specific differences in drug distribution/metabolism, and differences in in vivo drug concentrations that are achievable, among others. These contribute to the observation of extremely poor correlation between results of murine studies and early human clinical trials with anticancer agents (1). More predictive animal models are clearly needed.
More than 1 million new cases of cancer are thought to occur in dogs each year in the United States, and in retrospective studies describing canine mortality, cancer is the most common cause of death with an estimated rate of ∼30% (2–4). This large cancer burden in dogs indicates a group of spontaneously occurring tumors, many of which are histologically similar to human tumors. Commonly encountered histotypes include non-Hodgkin lymphoma, malignant melanoma, osteosarcoma (OSA), bladder carcinoma, and multiple brain cancer types among others (2, 5). Client-owned dogs with cancer are being increasingly recognized as a resource for preclinical interrogation of the tolerability, pharmacology, pharmacodynamic effects, and potential efficacy of novel anticancer therapies. This model’s potential was discussed in a National Academy of Medicine Workshop on Comparative Oncology that occurred in 2015 (http://www.nap.edu/21830) (6).
Clinical trials in client-owned dogs with spontaneous cancer are potentially important translational models, owing to dogs’ relative outbreeding, large size, immunocompetence, and physiological/biological similarity to humans. Spontaneous canine tumors naturally develop treatment resistance, as well as spontaneous recurrence and metastasis. Absolute tumor burdens in dogs are more similar to humans, which may be informative with regard to biological factors such as clonal heterogeneity and hypoxia. The comparatively large size of canine tumors (vs. rodent tumors) also allows for serial tissue collection and imaging over time (2, 7). This is due partly to the fact that these patients are commonly sedated or anesthetized for procedures, moderating concerns over patient discomfort. While clinical case management and data collection are of very high quality, the relative cost of veterinary oncology clinical trials are 10–20% of what similar trials in physician-based oncology would be.
Dogs may also be more reliable models for assessing toxicity of novel therapies than are rodents. As in human patients, canine patients are monitored for hematologic and biochemical toxicities via routine clinical pathology, and sophisticated monitoring (e.g., 24 hour continuous electrocardiographic telemetry, continuous blood pressure measurement, ophthalmologic monitoring, echocardiography, gait analysis, advanced imaging) can be performed as-needed. Unlike in laboratory settings, supportive care (e.g., antiemetics, antidiarrheals, antibiotics, etc.) is also used in client-owned animals similarly to its employment in human patients. Universally accepted grading systems for adverse events from antineoplastic therapy (8, 9), as well as universally accepted tumor response criteria (10, 11), are published.
The Comparative Oncology Trials Consortium (COTC: https://ccr.cancer.gov/Comparative- Oncology- Program/sponsors/consortium) is a network of more than 20 academic veterinary oncology centers, centrally managed by the Comparative Oncology Program, housed within the NIH-NCI-Center for Cancer Research. Its central goal is to plan and perform clinical trials in dogs with cancer to evaluated novel potential therapies for human cancer, with the goal of answering biological questions to inform development for future human clinical trials. COTC sponsored trials are usually pharmacokinetically/pharmacodynamically intensive, with the results incorporated into the design of future human studies. The launch of this network has improved the ability of potential sponsors and collaborators to access a national cooperative group for the conduct of proof of concept studies in dogs. Potential sponsors work with COTC management to iteratively develop a clinical protocol to address a specific drug development question/questions, which is then put out to the membership for potential participation. COTC sites have the opportunity to participate or decline based on capacity, specialized equipment/techniques that may be required, and/or competing trials at the institution. Trial conduct is governed by a single memorandum of understanding between the participating sites, which streamlines the contractual process.
These important attributes have allowed the preclinical evaluation of novel cancer therapeutics that fall into several broad categories: (1) Local therapy approaches such as surgery,
radiation therapy and locally-delivered drug therapy; (2) Proof of target inhibition and proof of tumor targeting; (3) Studies in the minimal residual disease setting; (4) Immunotherapy studies.
LOCAL THERAPY APPROACHES
As a result of dogs’ comparatively large body size and the relative size of their tumors, tumor-bearing dogs can be a unique and informative model for the evaluation of novel local therapies. Surgical and radiation therapy (RT) related studies can utilize the same techniques and equipment as would be used in human patients, without the need for the significant adaptation or miniaturization which could be required for rodents. As stated above, the comparatively similar size and growth rate of dog tumors results in similarities in important microenvironmental parameters such as oxygenation, pH, and interstitial fluid pressure (12–18), and the large tumor size facilitates serial biopsy and measurement of intratumoral parameters over time. As a result, tumor-bearing dogs have been utilized in translational studies of novel surgical approaches, RT, hyperthermia, and regionally-delivered drug therapy.
Translational Surgical Studies
National Cancer Institute sponsored work by Withrow et al. in the 1980’s pioneered surgical protocols for cortical allografts for limb-salvage in bone sarcoma patients. These procedures were co-developed by veterinary and human surgical oncologists and refined in a large number of dogs with spontaneous OSA, mostly of the distal radius. Effects of neoadjuvant RT and chemotherapy on surgical outcome and allograft incorporation were also assessed (19–21). These observations and subsequent refinements developed in dogs led directly to the use of these approaches in human limb-sparing surgery (22). An observation was made regarding the postoperative development of bacterial osteomyelitis and improved metastasis-free and overall survival times in dogs (23). This was subsequently observed in at least one study of humans with OSA (24). Further study of this observation in a murine syngeneic OSA model suggested NK- and monocyte- mediated angiogenesis inhibition as a putative mechanism of action (25).
Radiation Therapy
Studies of radiation by Gillette et al. in the 1970’s and 1980’s in both normal and tumor-bearing dogs established many normal tissue RT dose constraints still in use today in human patients (19, 26–37). More recently, studies in tumor-bearing dogs provided critical proof of concept for accurate dosimetry and conformal avoidance during the development of helical tomotherapy, a slice-by-slice image-guided intensity modulated RT strategy that is now commercially available (38, 39).
Translational Studies of Hyperthermia and
Radiation Therapy
A substantial body of literature documents pioneering NCI- funded work by Dewhirst et al. evaluating the effects of hyperthermia and hyperthermia/RT combinations on the tumor microenvironment in canine tumors, especially soft-tissue
sarcomas. As a result of their common subcutaneous location and the relative ease with which procedures such as serial biopsy and interstitial probe placement can be performed, meaningful insights into thermal dosimetry, alterations in tumor perfusion and tumor oxygenation, and predictors of clinical response were identified (36, 40–42).
Locally/Regionally Delivered Therapeutics
Multiple studies of inhaled/pulmonary delivered therapeutics have evaluated safety and provided preliminary evidence of antitumor efficacy in support of human trials. These include evaluation of inhaled doxorubicin, paclitaxel and gemcitabine for the treatment of measurable primary or metastatic pulmonary tumors (43, 44), and nebulized inhaled interleukin-2 (IL-2) containing liposomes for treatment of pulmonary metastatic OSA (45). In addition to the observed objective antitumor responses, the latter study included serially collected bronchoalveolar lavage (BAL) fluid to characterize the local leukocyte population before and after IL-2 therapy. Post- IL-2 BAL samples contained a more than four-fold increase in lymphocytes, with a shifted CD4:CD8 ratio and increased cytolytic activity ex vivo (45).
Various intratumor treatments have been evaluated in tumor- bearing dog models. These include attenuated Clostridium spores (46), and various intralesional gene therapy approaches (47–50). In many of these studies, serial biopsy was performed to evaluate and characterize immune infiltrates and/or confirm transgene expression. Several novel intralesional chemotherapy approaches (± other local treatments such as RT or hyperthermia) have likewise been evaluated, demonstrating tolerability and preliminary evidence of efficacy (50–55).
PROOF OF TARGET INHIBITION OR PROOF OF TUMOR TARGETING/ACCUMULATION
Owing again to the relative ease of serial biopsy, as well as comparably favorable pharmacokinetic parameters in dogs such as organ-specific blood flow and hepatic enzyme homologies, canine tumors can serve as very useful translational models for evaluation of pharmacokinetic/pharmacodynamic (PK/PD) relationships, demonstration of target inhibition, and/or demonstration of tumor targeting. In these cases, substantial preliminary in vitro work is often necessary to confirm target expression, demonstrate similar drug behavior in canine and human tumor cells, and potentially validate reagents and protocols necessary for PD assessment. Importantly, there are certain situations where molecular targets may be present in canine tumors that are histologically very different from human tumors expressing the same target. Examples include expression of mutant KIT protein in canine mast cell tumors (MCT) with a similar mutation expressed in human gastrointestinal stromal tumors (56), and expression of the V600E BRAF mutation, commonly expressed in human melanomas, in canine bladder cancer (57, 58).
Proof of Drug Target Inhibition
An early example of successful evaluation of a novel targeted agent in dogs with spontaneous neoplasia involves the preclinical evaluation of the “split kinase” inhibitor SU11654 (toceranib phosphate, PalladiaTM), in dogs with MCT. SU11654 is a structural analog of the human multi-kinase inhibitor sunitinib (SutentTM) with very similar physicochemical properties and IC50’s against their intended targets, which include KIT, VEGFR2 and PDGFR-alpha. After initial in vitro studies demonstrating canine MCT growth inhibition, apoptosis induction and inhibition of KIT phosphorylation (59), pilot studies were performed in tumor-bearing dogs demonstrating achievement of likely therapeutic drug concentrations in plasma with good tolerability and evidence of antitumor activity (60). Furthermore, inhibition of KIT activation and downstream signaling was demonstrated in biopsy samples prior to and 8 h following the first dose of drug (61). These data provided critical information in support of the human development of sunitinib, which is now approved by the U.S. Food and Drug Administration (FDA) for human renal cell carcinoma, pancreatic neuroendocrine tumors, and gastrointestinal stromal tumors, and led to the FDA approval of toceranib for the treatment of canine MCT (62).
A similar “next to lead” approach has been taken with the selective inhibitor of nuclear export verdinexor (KPT-335), which was evaluated in vitro for activity in canine tumor cells, then in tumor-bearing dogs to provide supporting data for development of the human analog selinexor (KPT-330, XpovioTM) (63), now approved by the FDA for the treatment of human multiple myeloma. Verdinexor is now in clinical development as a canine cancer therapeutic.
Rather than evaluating a structural analog to generate preclinical data in tumor-bearing dogs in support of a human clinical candidate, another recent study evaluated PCI-32765
(ibrutinib, ImbruvicaTM ), a first-in-class inhibitor of the Bruton tyrosine kinase (Btk), in dogs with spontaneous B-cell lymphoma prior to first-in-in-human studies (64). Goals of the study were 2- fold: (1) To validate a PD assay to be used in subsequent human trials; (2) To generate preliminary evidence of efficacy, since reliable murine B-cell lymphoma models demonstrating intact B cell receptor signaling were not available. Btk receptor occupancy following ibrutinib treatment was similar in lymphoma tissue and peripheral blood following treatment, providing support that measurement in blood alone would likely be accurate in humans. Furthermore, major antitumor responses were observed in three of eight dogs treated, providing strong impetus to accelerate human clinical development of ibrutinib. Ibrutinib now has FDA approval in humans for the treatment of certain B cell lymphoma subtypes, chronic lymphocytic leukemia, Waldenstrom’s macroglobulinemia and graft-vs.-host disease.
Proof of Tumor Targeting
Canine tumors have been utilized to confirm tumor-specific targeting and/or tumor accumulation of therapeutics. The inaugural COTC trial evaluated a tumor vasculature targeted adeno-associated virus phage vector targeted to alphaV integrins expressed on tumor endothelium and delivering tumor necrosis factor (TNF), in preparation
for human trials. Selective targeting of tumor (vs. normal) vasculature was documented through serial biopsy of tumor and proximate normal tissues after intravenous infusion, and tumor-directed expression of TNF was documented. Furthermore, objective antitumor responses were noted in 2 of 14 dogs (65).
Certain bacteria, especially facultative anaerobes, demonstrate tropism for tumor tissues. VNP20009 is a Salmonella typhimurium strain that was attenuated through deletion of the MsbB gene, contributing to endotoxin production, and the PurI gene, requiring an exogenous source of purines for survival. These deletions reduce toxicity and further restrict colonization to tumor tissues in vivo, while diminishing or eliminating survival in the environment. Intravenous infusion of VNP20009 was evaluated in tumor-bearing dogs for safety and evidence of tumor colonization (66). While blood cultures were uniformly negative 7 days following infusion, the organism was isolated from tumor tissue in 42% of dogs. The objective response rate was 15% (10% complete responses). These data supported an NCI-sponsored clinical trial of VNP20009 in human metastatic melanoma (67). No objective antitumor responses were observed in the human melanoma study, however; this could be due to selection of melanoma as the sole human tumor type for study, or due to differences in either tolerability or host (e.g., immune, vascular) response to the bacterium between dog and human. Strategies for geographically targeted cytotoxic drug delivery via hyperthermia and thermosensitive liposomes have also been investigated in canine soft tissue sarcomas (68).
Proof of Tumor Drug Accumulation
A recent canine clinical trial of the autophagy modulating agent hydroxychloroquine (HCQ), which was published concurrently with a series of human clinical trials, was the first to document substantial accumulation (∼100-fold) of HCQ in tumor tissue when compared with plasma, and to demonstrate that there was no correlation between drug concentrations or changes in autophagy in the two compartments. This suggested that peripheral blood is not a good surrogate for tumor HCQ concentration or autophagy-modulatory activity, and that future clinical trials should aim to identify more accurate surrogates of HCQ activity (69).
Another large COTC trial evaluated a series of three distinct indenoisoquinolone-class topoisomerase I inhibitors in dogs with spontaneous lymphoma. Eighty-four dogs with lymphoma were allocated to receive one of three drugs. Tolerability, pharmacokinetics, target engagement and antitumor effects were evaluated. One of the three drugs, LMP744, demonstrated significantly increased accumulation in tumor tissue vs. the other two drugs, and enhanced antitumor activity was ascribed to this increased tumor accumulation (70). Although LMP744 was not originally selected for further human development, the unexpected positive results of the canine trial encouraged the NCI to evaluate LMP744 in humans (ClinicalTrials.gov identifier NCT03030417). This human trial is currently accruing and thus human safety/efficacy data are not currently available.
ANTIMETASTATIC EFFICACY
Another potential advantage of canine clinical cancer research is the relatively compressed time line for tumor progression and the spontaneous development of local recurrence, metastasis, and drug resistance. These characteristics allow surgical adjuvant studies against “microscopic residual disease,” with temporal endpoints such as progression free or overall survival, to be conducted relatively expediently. This may be useful especially for agents designed primarily as antimetastatic therapies. Several candidate human therapies have been investigated in this context in tumor-bearing dogs.
Extensive work by Macewen, Kurzman et al. with the peptidoglycan recognition protein agonist and non- specific immune stimulant liposome muramyl tripeptide phosphatidylethanolamine (L-MTP-PE) was performed in dogs with hemangiosarcoma (HSA) and OSA. Randomized placebo controlled trials demonstrated meaningful delays in metastasis and prolongation of overall survival times when surgery and chemotherapy were combined with L-MTP-PE (71, 72). Furthermore, bronchoalveolar lavage performed before and after L-MTP-PE indicated significant enhancement of activation status and ex vivo antitumor cytotoxicity in pulmonary alveolar macrophages (73). This work provided critical proof of principle showing delay of metastasis in OSA, which led directly to the performance of a randomized, placebo-controlled trial of surgery, chemotherapy ± L-MTP-PE in human OSA (74).
Subsequently, L-MTP-PE (mifamurtide, MepactTM ) was granted regulatory approval by the European Medicines Agency for treatment of human OSA.
Another randomized, multicenter surgical adjuvant study compared standard-of-care therapy with carboplatin to treatment with the novel liposomal cisplatin drug SPI-77 in dogs with appendicular OSA. Despite SPI-77’s capacity to deliver five times more cisplatin vs. the maximum tolerated dose of free cisplatin, there were no improvements in progression free survival time or overall survival time when compared with conventionally dosed carboplatin. These results, combined with other factors, contributed significantly to the decision to suspend SPI-77’s clinical development (75).
In a recent study, dogs with splenic HSA were treated after splenectomy with a combination of doxorubicin and an epidermal growth factor receptor- and urokinase-targeted Pseudomonas exotoxin, referred to as eBAT. These targets appear to be conserved in certain human sarcomas, and thus canine HSA may be a valuable translational model despite the distinct histotype and rareness of its human homolog. In addition to very good tolerability, there was the suggestion of improved outcome when eBAT-treated patients were compared with historical canine patients receiving doxorubicin alone (76). The human development path for eBAT is not currently known.
IMMUNOTHERAPY
In addition to the advantages discussed above, a unique advantage of spontaneous canine tumors that has been somewhat overlooked is that these tumors have evolved, by necessity,
immune-avoidance strategies that are very similar to those utilized by human cancers. This is in stark contrast to syngeneic murine tumor models, where immune tolerance does not evolve similarly. These immune-avoidance strategies include upregulation of immune-suppressive cytokines such as IL-8, IL-10, and transforming growth factor beta (77–80), cooptation of innate immune-suppressive cells such as regulatory T cells (81–83), myeloid-derived suppressor cells (84–86), and “steady state” macrophages (87–90), and upregulation of immune checkpoint molecules such as PD-L1 and B7x (91–95). Thus, successful cancer immunotherapy in dogs requires overcoming of these conserved immune-avoidance strategies just as is required in humans.
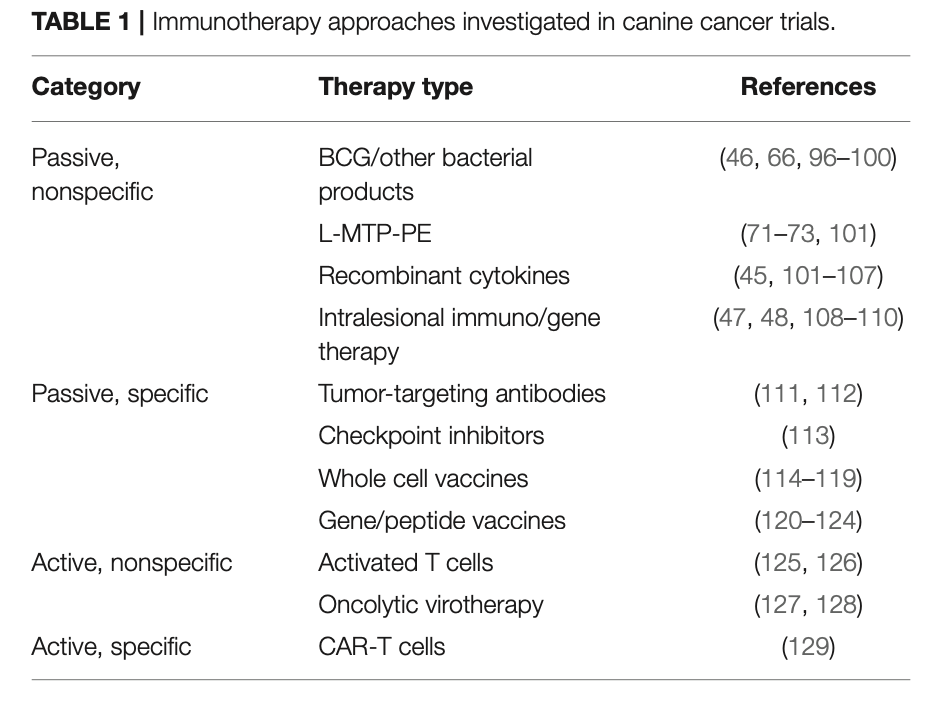
In addition to the approaches mentioned in previous sections, a variety of immunotherapy strategies have been investigated in dogs over decades. These range from passive non-specific immunotherapy approaches to early studies with canine chimeric antigen receptor-engineered T (CAR-T) cells. A partial list of immunotherapy approaches investigated in dogs with cancer is provided in Table1. An exhaustive discussion of these approaches is beyond the scope of this review; however, this issue contains a dedicated article discussing canine tumor immunology and immunotherapy. Several of the approaches outlined in the Table have led to human clinical trials (74, 130, 131).
CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion, there is great potential for studies in dogs with spontaneous cancer to inform development of novel human therapeutics and diagnostics. In general, these studies have a higher potential for success when there is a focused, a priori question that canine studies seek to answer, and a plan for utilization of the data generated is in place prior to study commencement. Additionally, utilizing the strengths of the model, especially vis a vis the ability to repeatedly sample tumor tissue, to generate robust PK/PD related data is value-added.
These types of data are perhaps more critical in answering questions regarding why a treatment did not work than in supporting how a treatment did work. Was it an issue of insufficient drug exposure? Was there adequate exposure in plasma but not tumor? Was the target appropriately inhibited despite a lack of antitumor activity?
Additionally, successful implementation of studies in dogs generally requires some amount of preclinical work for validation of target expression, validation of drug activity against the canine analog of the target, and selection/validation of PD endpoints to be implemented in subsequent canine clinical trials. A lack of canine-specific reagents often requires some legwork for the validation of cross-reactive antibodies for these types of applications.
Ongoing foundational work has the potential to significantly expand the molecular underpinnings of canine cancer, and facilitate comparisons with human cancer. A number of 1 year administrative supplements to existing NIH P30 grants were recently approved, with the goals of utilizing next-gen sequencing (whole-exome sequencing, RNASeq) to characterize a variety of canine tumor types for quantification of mutational load, identification of driver mutations, and characterization of potential neoantigens for MHC binding. Furthermore, a series of U01 grants were recently funded by the NIH to explore novel immunotherapy approaches in canine cancer to inform human cancer immunotherapy studies. These studies have the potential to expand understanding of the molecular drivers of canine cancer and uncover novel shared molecular targets and pathways for future study.
Several ongoing large-scale longitudinal studies are taking advantage of dogs’ foreshortened life spans to answer a variety of questions about life style, environment, aging and cancer incidence, as well as evaluating novel interventions. The Golden Retriever Lifetime Health Study (www.morrisanimalfoundation. org/golden-retriever-lifetime-study) is following 3,000 US golden retrievers from young adulthood to death, to identify environmental, nutritional, genetic, and lifestyle risk factors for cancer and other diseases in dogs. The University of Washington Dog Aging Project (https://dogagingproject.org) seeks to profile and follow up to 10,000 dogs to determine incidence and risk factors for a variety of age-related diseases, as well as pursuing smaller-scale trials with novel anti-aging (and potentially anti- cancer) interventions. The Vaccination Against Canine Cancer Study (www.vaccs.org) is an 800-dog, randomized, placebo- controlled, prospective, multi-center clinical trial seeking to evaluate the evaluate the ability of a multivalent frameshift vaccine to delay or prevent cancer development in healthy older dogs. These three long-term studies have the potential to shed significant light on genetic, environmental, lifestyle, and immunologic risk factors for cancer that may have significant translatability. The results are eagerly anticipated.
AUTHOR CONTRIBUTIONS
The author confirms being the sole contributor of this work and has approved it for publication.
REFERENCES
- Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. (2001) 84:1424–31. doi: 10.1054/bjoc.2001.1796
- Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. (2008) 8:147–56. doi: 10.1038/nrc2273
- Fleming JM, Creevy KE, Promislow DE. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. (2011) 25:187–98. doi: 10.1111/j.1939-1676.2011.0695.x
- Alvarez CE. Naturally occurring cancers in dogs: insights for translational genetics and medicine. ILAR J. (2014) 55:16–45. doi: 10.1093/ilar/ilu010
- Leblanc AK, Mazcko CN, Khanna C. Defining the value of a comparative
approach to cancer drug development. Clin Cancer Res. (2016) 22:2133–8.
doi: 10.1158/1078-0432.CCR-15-2347
- Leblanc AK, Breen M, Choyke P, Dewhirst M, Fan TM, Gustafson DL, et
al. Perspectives from man’s best friend: National Academy of Medicine’s Workshop on Comparative Oncology. Sci Transl Med. (2016) 8:324ps325. doi: 10.1126/scitranslmed.aaf0746
- Vail DM, Thamm DH. Spontaneously occurring tumors in companion animals as models for drug development. In: Teicher BA, Andrews PA, editors. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval, 2nd ed. Totowa, NJ: Humana Press (2004). p. 259–84.
- Ladue T, Klein MK, Veterinary Radiation Therapy Oncology G. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound. (2001) 42:475–6. doi: 10.1111/j.1740-8261.2001.tb00973.x
- Veterinary Cooperative Oncology Group. Veterinary Cooperative Oncology Group – common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. (2016) 14:417–46. doi: 10.1111/vco.283
- Vail DM, Michels GM, Khanna C, Selting KA, London CA, Veterinary Cooperative Oncology G. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)–a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. (2010) 8:28–37. doi: 10.1111/j.1476-5829.2009.00200.x
- Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. (2015) 13:176–83. doi: 10.1111/vco.12032
- Cline JM, Thrall DE, Page RL, Franko AJ, Raleigh JA. Immunohistochemical detection of a hypoxia marker in spontaneous canine tumours. Br J Cancer. (1990) 62:925–31. doi: 10.1038/bjc.1990.411
- Zeman EM, Calkins DP, Cline JM, Thrall DE, Raleigh JA. The relationship between proliferative and oxygenation status in spontaneous canine tumors. Int J Radiat Oncol Biol Phys. (1993) 27:891–8. doi: 10.1016/0360-3016(93)90465-8
- Cline JM, Thrall DE, Rosner GL, Raleigh JA. Distribution of the hypoxia marker CCI-103F in canine tumors. Int J Radiat Oncol Biol Phys. (1994) 28:921–33. doi: 10.1016/0360-3016(94)90113-9
- Raleigh JA, Zeman EM, Calkins DP, Mcentee MC, Thrall DE. Distribution of hypoxia and proliferation associated markers in spontaneous canine tumors. Acta Oncol. (1995) 34:345–9. doi: 10.3109/02841869509093987
- Cline JM, Rosner GL, Raleigh JA, Thrall DE. Quantification of CCI-103F labeling heterogeneity in canine solid tumors. Int J Radiat Oncol Biol Phys. (1997) 37:655–62. doi: 10.1016/S0360-3016(96)00559-7
- Thrall DE, Rosner GL, Azuma C, Mcentee MC, Raleigh JA. Hypoxia marker labeling in tumor biopsies: quantification of labeling variation and criteria for biopsy sectioning. Radiother Oncol. (1997) 44:171–6. doi: 10.1016/S0167-8140(97)01931-2
- Zachos TA, Aiken SW, Diresta GR, Healey JH. Interstitial fluid pressure and blood flow in canine osteosarcoma and other tumors. Clin Orthop Relat Res. (2001) 230–6. doi: 10.1097/00003086-200104000- 00034
- Larue SM, Withrow SJ, Powers BE, Wrigley RH, Gillette EL, Schwarz PD, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc. (1989) 195:1734–44.
20.
21.
22. 23.
24.
25.
26. 27.
28. 29.
30. 31.
32.
33. 34. 35. 36.
37. 38. 39.
40.
Thrall DE, Withrow SJ, Powers BE, Straw RC, Page RL, Heidner GL, et al. Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: a dose response assay. Int J Radiat Oncol Biol Phys. (1990) 18:1351–7. doi: 10.1016/0360-3016(90)90308-7
Withrow SJ, Thrall DE, Straw RC, Powers BE, Wrigley RH, Larue SM, et al. Intra-arterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer. (1993) 71:2484–90. doi: 10.1002/1097- 0142(19930415)71:8<2484::AID-CNCR2820710810>3.0.CO;2-D
Withrow SJ, Wilkins RM. Cross talk from pets to people: translational osteosarcoma treatments. ILAR J. (2010) 51:208–13. doi: 10.1093/ilar.51.3.208
Lascelles BD, Dernell WS, Correa MT, Lafferty M, Devitt CM, Kuntz CA, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. (2005) 12:1073–83. doi: 10.1245/ASO.2005.01.011
Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. (2007) 14:2887–95. doi: 10.1245/s10434-007-9483-8
Sottnik JL, U’Ren LW, Thamm DH, Withrow SJ, Dow SW. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother. (2010) 59:367–78. doi: 10.1007/s00262-009-0755-y
Gillette EL, Maurer GD, Severin GA. Endothelial repair of radiation damage following beta irradiation. Radiology. (1975) 116:175–7. doi: 10.1148/116.1.175
Fike JR, Gillette EL, Clow DJ. Repair of sublethal radiation damage by capillaries. Int J Radiat Oncol Biol Phys. (1979) 5:339–42. doi: 10.1016/0360-3016(79)91213-6
Gavin PR, Gillette EL. Radiation response of the canine cardiovascular system. Radiat Res. (1982) 90:489–500. doi: 10.2307/3575726
Gillette EL, Mcchesney SL, Hoopes PJ. Isoeffect curves for radiation-induced cardiomyopathy in the dog. Int J Radiat Oncol Biol Phys. (1985) 11:2091–7. doi: 10.1016/0360-3016(85)90089-6
Hoopes PJ, Gillette EL, Benjamin SA. The pathogenesis of radiation nephropathy in the dog. Radiat Res. (1985) 104:406–19. doi: 10.2307/3576600 Powers BE, Mcchesney SL, Gillette EL. Late radiation response of the canine trachea with change in dose per fraction. Int J Radiat Oncol Biol Phys. (1987) 13:1673–80. doi: 10.1016/0360-3016(87)90164-7
Ahmadu-Suka F, Gillette EL, Withrow SJ, Husted PW, Nelson AW, Whiteman CE. Exocrine pancreatic function following intraoperative irradiation of the canine pancreas. Cancer. (1988) 62:1091–5. doi: 10.1002/ 1097-0142(19880915)62:6<1091::AID-CNCR2820620611>3.0.CO;2-A Ching SV, Gillette SM, Powers BE, Roberts SM, Gillette EL, Withrow SJ. Radiation-induced ocular injury in the dog: a histological study. Int J Radiat Oncol Biol Phys. (1990) 19:321–8. doi: 10.1016/0360-3016(90)90540-Z Gillette SM, Powers BE, Orton EC, Gillette EL. Early radiation response of the canine heart and lung. Radiat Res. (1991) 125:34–40. doi: 10.2307/3577979
Powers BE, Gillette EL, Gillette SL, Lecouteur RA, Withrow SJ. Muscle injury following experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys. (1991) 20:463–71. doi: 10.1016/0360-3016(91)90058-C
Gillette SM, Dewhirst MW, Gillette EL, Thrall DE, Page RL, Powers BE, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia: a randomized phase II study. Int J Hyperthermia. (1992) 8:309–20. doi: 10.3109/02656739209021786
McChesney SL, Gillette EL, Powers BE. Response of the canine lung to fractionated irradiation: pathologic changes and isoeffect curves. Int J Radiat Oncol Biol Phys. (1989) 16:125–32. doi: 10.1016/0360-3016(89)90019-9 Mackie TR, Kapatoes J, Ruchala K, Lu W, Wu C, Olivera G, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys. (2003) 56:89–105. doi: 10.1016/S0360-3016(03)00090-7
Forrest LJ, Mackie TR, Ruchala K, Turek M, Kapatoes J, Jaradat H, et al. The utility of megavoltage computed tomography images from a helical tomotherapy system for setup verification purposes. Int J Radiat Oncol Biol Phys. (2004) 60:1639–44. doi: 10.1016/j.ijrobp.2004.08.016
Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in
spontaneous canine sarcomas. Int J Hyperthermia. (2006) 22:365–73.
doi: 10.1080/02656730600836386
- Chi JT, Thrall DE, Jiang C, Snyder S, Fels D, Landon C, et al.
Comparison of genomics and functional imaging from canine sarcomas treated with thermoradiotherapy predicts therapeutic response and identifies combination therapeutics. Clin Cancer Res. (2011) 17:2549–60. doi: 10.1158/1078-0432.CCR-10-2583
- Thrall DE, Maccarini P, Stauffer P, Macfall J, Hauck M, Snyder S, et al. Thermal dose fractionation affects tumour physiological response. Int J Hyperthermia. (2012) 28:431–40. doi: 10.3109/02656736.2012.689087
- Hershey AE, Kurzman ID, Forrest LJ, Bohling CA, Stonerook M, Placke ME, et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. (1999) 5:2653–9.
- Rodriguez CO Jr., Crabbs TA, Wilson DW, Cannan VA, Skorupski KA, et al. Aerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J Aerosol Med Pulm Drug Deliv. (2010) 23:197–206. doi: 10.1089/jamp.2009.0773
- Khanna C, Anderson PM, Hasz DE, Katsanis E, Neville M, Klausner JS. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer. (1997) 79:1409–21. doi: 10. 1002/(SICI)1097-0142(19970401)79:7<1409::AID-CNCR19>3.0.CO;2-3
- Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. (2014) 6:249ra111. doi: 10.1126/scitranslmed.3008982
- Dow SW, Elmslie RE, Willson AP, Roche L, Gorman C, Potter TA. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J Clin Invest. (1998) 101:2406–14. doi: 10.1172/JCI510
- Thamm DH, Kurzman ID, Macewen EG, Feinmehl R, Towell TL, Longhofer SL, et al. Intralesional lipid-complexed cytokine/superantigen immunogene therapy for spontaneous canine tumors. Cancer Immunol Immunother. (2003) 52:473–80. doi: 10.1007/s00262-003-0387-6
- Finocchiaro LM, Villaverde MS, Gil-Cardeza ML, Riveros MD, Glikin GC. Cytokine-enhanced vaccine and interferon-beta plus suicide gene as combined therapy for spontaneous canine sarcomas. Res Vet Sci. (2011) 91:230–4. doi: 10.1016/j.rvsc.2010.12.012
- Westberg S, Sadeghi A, Svensson E, Segall T, Dimopoulou M, Korsgren O, et al. Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J Immunother. (2013) 36:350–8. doi: 10.1097/CJI.0b013e31829d8a1b
- Theon AP, Madewell BR, Moore AS, Stephens C, Krag DN. Localized thermo-cisplatin therapy: a pilot study in spontaneous canine and feline tumours. Int J Hyperthermia. (1991) 7:881–92. doi: 10.3109/02656739109056456
- Kitchell BE, Brown DM, Luck EE, Woods LL, Orenberg EK, Bloch DA. Intralesional implant for treatment of primary oral malignant melanoma in dogs. J Am Vet Med Assoc. (1994) 204:229–36.
- Theon AP, Madewell BR, Ryu J, Castro J. Concurrent irradiation and intratumoral chemotherapy with cisplatin: a pilot study in dogs with spontaneous tumors. Int J Radiat Oncol Biol Phys. (1994) 29:1027–34. doi: 10.1016/0360-3016(94)90398-0
- Kitchell BK, Orenberg EK, Brown DM, Hutson C, Ray K, Woods L, et al. Intralesional sustained-release chemotherapy with therapeutic implants for treatment of canine sun-induced squamous cell carcinoma. Eur J Cancer. (1995) 31A:2093–8. doi: 10.1016/0959-8049(95)00446-7
- Venable RO, Worley DR, Gustafson DL, Hansen RJ, Ehrhart EJ III, Cai S, et al. Effects of intratumoral administration of a hyaluronan-cisplatin nanoconjugate to five dogs with soft tissue sarcomas. Am J Vet Res. (2012) 73:1969–76. doi: 10.2460/ajvr.73.12.1969
- London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp Hematol. (1999) 27:689–97. doi: 10.1016/S0301-472X(98) 00075-7
- Decker B, Parker HG, Dhawan D, Kwon EM, Karlins E, Davis BW, et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer–evidence for a relevant model system
58. 59.
60.
61. 62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
and urine-based diagnostic test. Mol Cancer Res. (2015) 13:993–1002. doi: 10.1158/1541-7786.MCR-14-0689
Mochizuki H, Breen M. Sequence analysis of RAS and RAF mutation hot spots in canine carcinoma. Vet Comp Oncol. (2017) 15:1598–605. doi: 10.1111/vco.12275
Liao AT, Chien MB, Shenoy N, Mendel DB, Mcmahon G, Cherrington JM, et al. Inhibition of constitutively active forms of mutant kit by multitargeted indolinone tyrosine kinase inhibitors. Blood. (2002) 100:585– 93. doi: 10.1182/blood-2001-12-0350
London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. (2003) 9:2755–68.
Pryer NK, Lee LB, Zadovaskaya R, Yu X, Sukbuntherng J, Cherrington JM, et al. Proof of target for SU11654: inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. (2003) 9:5729–34.
London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. (2009) 15:3856–65. doi: 10.1158/1078-0432.CCR-08-1860
London CA, Bernabe LF, Barnard S, Kisseberth WC, Borgatti A, Henson M, et al. Preclinical evaluation of the novel, orally bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PLoS ONE. (2014) 9:e87585. doi: 10.1371/journal.pone.0087585
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. (2010) 107:13075–80. doi: 10.1073/pnas.1004594107 Paoloni MC, Tandle A, Mazcko C, Hanna E, Kachala S, Leblanc A, et al. Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PLoS ONE. (2009) 4:e4972. doi: 10.1371/journal.pone.0004972 Thamm DH, Kurzman ID, King I, Li Z, Sznol M, Dubielzig RR, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res. (2005) 11:4827–34. doi: 10.1158/1078-0432.CCR-04-2510
Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. (2002) 20:142–52. doi: 10.1200/JCO.20.1.142
Hauck ML, Larue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res. (2006) 12:4004– 10. doi: 10.1158/1078-0432.CCR-06-0226
Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. (2014) 10:1415– 25. doi: 10.4161/auto.29165
Burton JH, Mazcko C, Leblanc A, Covey JM, Ji J, Kinders RJ, et al. NCI Comparative Oncology Program testing of non- camptothecin indenoisoquinoline topoisomerase i inhibitors in naturally occurring canine lymphoma. Clin Cancer Res. (2018) 24:5830–40. doi: 10.1158/1078-0432.CCR-18-1498
Kurzman ID, Macewen EG, Rosenthal RC, Fox LE, Keller ET, Helfand SC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. (1995) 1:1595–601.
Vail DM, Macewen EG, Kurzman ID, Dubielzig RR, Helfand SC, Kisseberth WC, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multi-institutional clinical trial. Clin Cancer Res. (1995) 1:1165–70.
Kurzman ID, Shi F, Vail DM, Macewen EG. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity
against canine osteosarcoma cells. Cancer Biother Radiopharm. (1999)
14:121–8. doi: 10.1089/cbr.1999.14.121
- Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D,
Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. (2005) 23:2004–11. doi: 10.1200/JCO.2005.06.031
- Vail DM, Kurzman ID, Glawe PC, O’Brien MG, Chun R, Garrett LD, et al. STEALTH liposome-encapsulated cisplatin (SPI-77) versus carboplatin as adjuvant therapy for spontaneously arising osteosarcoma (OSA) in the dog: a randomized multicenter clinical trial. Cancer Chemother Pharmacol. (2002) 50:131–6. doi: 10.1007/s00280-002-0469-8
- Borgatti A, Koopmeiners JS, Sarver AL, Winter AL, Stuebner K, Todhunter D, et al. Safe and effective sarcoma therapy through bispecific targeting of EGFR and uPAR. Mol Cancer Ther. (2017) 16:956–65. doi: 10.1158/1535-7163.MCT-16-0637
- Itoh H, Horiuchi Y, Nagasaki T, Sakonju I, Kakuta T, Fukushima U, et al. Evaluation of immunological status in tumor-bearing dogs. Vet Immunol Immunopathol. (2009) 132:85–90. doi: 10.1016/j.vetimm.2009.04.020
- De Andres PJ, Illera JC, Caceres S, Diez L, Perez-Alenza MD, Pena L. Increased levels of interleukins 8 and 10 as findings of canine inflammatory mammary cancer. Vet Immunol Immunopathol. (2013) 152:245–51. doi: 10.1016/j.vetimm.2012.12.010
- Kim JH, Frantz AM, Anderson KL, Graef AJ, Scott MC, Robinson S, et al. Interleukin-8 promotes canine hemangiosarcoma growth by regulating the tumor microenvironment. Exp Cell Res. (2014) 323:155–64. doi: 10.1016/j.yexcr.2014.02.020
- Troyer RM, Ruby CE, Goodall CP, Yang L, Maier CS, Albarqi HA, et al. Exosomes from osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res. (2017) 358:369–76. doi: 10.1016/j.yexcr.2017.07.011
- Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. (2007) 116:69–78. doi: 10.1016/j.vetimm.2006.12.002
- O’Neill K, Guth A, Biller B, Elmslie R, Dow S. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med. (2009) 23:875–81. doi: 10.1111/j.1939-1676.2009.0333.x
- Biller BJ, Guth A, Burton JH, Dow SW. Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med. (2010) 24:1118–23. doi: 10.1111/j.1939-1676.2010.0557.x
- Goulart MR, Pluhar GE, Ohlfest JR. Identification of myeloid derived suppressor cells in dogs with naturally occurring cancer. PLoS ONE. (2012) 7:e33274. doi: 10.1371/journal.pone.0033274
- Sherger M, Kisseberth W, London C, Olivo-Marston S, Papenfuss TL. Identification of myeloid derived suppressor cells in the peripheral blood of tumor bearing dogs. BMC Vet Res. (2012) 8:209. doi: 10.1186/1746-6148-8-209
- Goulart MR, Hlavaty SI, Chang YM, Polton G, Stell A, Perry J, et al. Phenotypic and transcriptomic characterization of canine myeloid-derived suppressor cells. Sci Rep. (2019) 9:3574. doi: 10.1038/s41598-019-40285-3
- Beirao BC, Raposo T, Pang LY, Argyle DJ. Canine mammary cancer cells direct macrophages toward an intermediate activation state between M1/M2. BMC Vet Res. (2015) 11:151. doi: 10.1186/s12917-015-0473-y
- Regan DP, Escaffi A, Coy J, Kurihara J, Dow SW. Role of monocyte recruitment in hemangiosarcoma metastasis in dogs. Vet Comp Oncol. (2017) 15:1309–22. doi: 10.1111/vco.12272
- Monteiro LN, Rodrigues MA, Gomes DA, Salgado BS, Cassali GD. Tumour- associated macrophages: relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Vet J. (2018) 234:119–25. doi: 10.1016/j.tvjl.2018.02.016
- Seung BJ, Lim HY, Shin JI, Kim HW, Cho SH, Kim SH, et al. CD204- expressing tumor-associated macrophages are associated with malignant, high-grade, and hormone receptor-negative canine mammary gland tumors. Vet Pathol. (2018) 55:417–24. doi: 10.1177/0300985817750457
- Maekawa N, Konnai S, Ikebuchi R, Okagawa T, Adachi M, Takagi S, et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-gamma
92.
93.
94. 95.
96.
97. 98.
99.
100.
101.
102. 103.
104.
105. 106.
107.
108.
production from tumor-infiltrating cells by PD-L1 blockade. PLoS ONE. (2014) 9:e98415. doi: 10.1371/journal.pone.0098415
Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE. (2016) 11:e0157176. doi: 10.1371/journal.pone.0157176
Ambrosius LA, Dhawan D, Ramos-Vara JA, Ruple A, Knapp DW, Childress MO. Quantification and prognostic value of programmed cell death ligand-1 expression in dogs with diffuse large B-cell lymphoma. Am J Vet Res. (2018) 79:643–9. doi: 10.2460/ajvr.79.6.643
Hartley G, Elmslie R, Dow S, Guth A. Checkpoint molecule expression by B and T cell lymphomas in dogs. Vet Comp Oncol. (2018) 16:352–60. doi: 10.1111/vco.12386
Chand SK, Pendharkar SA, Bharmal SH, Bartlett AS, Pandol SJ, Petrov MS. Frequency and risk factors for liver disease following pancreatitis: a population-based cohort study. Dig Liver Dis. (2019) 51:551–8. doi: 10.1016/j.dld.2018.11.001
Bech-Nielsen S, Brodey RS, Fidler IJ, Abt DA, Reif JS. The effect of BCG on in vitro immune reactivity and clinical course in dogs treated surgically for osteosarcoma. Eur J Cancer. (1977) 13:33–41. doi: 10.1016/0014-2964(77)90227-4
Bostock DE, Gorman NT. Intravenous BCG therapy of mammary carcinoma
in bitches after surgical excision of the primary tumour. Eur J Cancer. (1978) 14:879–83. doi: 10.1016/0014-2964(78)90104-4
Meyer JA, Dueland RT, Macewen EG, Macy DW, Hoefle WD, Richardson
RC, et al. Canine osteogenic sarcoma treated by amputation and MER:
an adverse effect of splenectomy on survival. Cancer. (1982) 49:1613–6.
doi: 10.1002/1097-0142(19820415)49:8<1613::AID-CNCR2820490814>3.0.CO;2- R
Parodi AL, Misdorp W, Mialot JP, Mialot M, Hart AA, Hurtrel M, et al. Intratumoral BCG and Corynebacterium parvum therapy of canine mammary tumours before radical mastectomy. Cancer Immunol Immunother. (1983) 15:172–7. doi: 10.1007/BF00199160
Henry CJ, Downing S, Rosenthal RC, Klein MK, Meleo K, Villamil JA, et al. Evaluation of a novel immunomodulator composed of human chorionic gonadotropin and bacillus Calmette-Guerin for treatment of canine mast cell tumors in clinically affected dogs. Am J Vet Res. (2007) 68:1246–51. doi: 10.2460/ajvr.68.11.1246
Macewen EG, Kurzman ID, Vail DM, Dubielzig RR, Everlith K, Madewell BR, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clin Cancer Res. (1999) 5:4249–58.
Moore AS, Theilen GH, Newell AD, Madewell BR, Rudolf AR. Preclinical study of sequential tumor necrosis factor and interleukin 2 in the treatment of spontaneous canine neoplasms. Cancer Res. (1991) 51:233–8.
Mito K, Sugiura K, Ueda K, Hori T, Akazawa T, Yamate J, et al. IFNγ markedly cooperates with intratumoral dendritic cell vaccine in dog tumor models. Cancer Res. (2010) 70:7093–101. doi: 10.1158/0008-5472.CAN-10-0600
Henson MS, Curtsinger JM, Larson VS, Klausner JS, Modiano JF, Mescher MF, et al. Immunotherapy with autologous tumour antigen-coated microbeads (large multivalent immunogen), IL-2 and GM-CSF in dogs with spontaneous B-cell lymphoma. Vet Comp Oncol. (2011) 9:95–105. doi: 10.1111/j.1476-5829.2010.00234.x
Konietschke U, Teske E, Jurina K, Stockhaus C. Palliative intralesional interleukin-2 treatment in dogs with urinary bladder and urethral carcinomas. In Vivo. (2012) 26:931–5.
Haagsman AN, Witkamp AC, Sjollema BE, Kik MJ, Kirpensteijn J. The effect of interleukin-2 on canine peripheral nerve sheath tumours after marginal surgical excision: a double-blind randomized study. BMC Vet Res. (2013) 9:155. doi: 10.1186/1746-6148-9-155
Ziekman PG, Otter WD, Tan JF, Teske E, Kirpensteijn J, Koten JW, et al. Intratumoural interleukin-2 therapy can induce regression of non-resectable mastocytoma in dogs. Anticancer Res. (2013) 33:161–5.
Finocchiaro LM, Fondello C, Gil-Cardeza ML, Rossi UA, Villaverde MS, Riveros MD, et al. Cytokine-enhanced vaccine and interferon-beta plus
suicide gene therapy as surgery adjuvant treatments for spontaneous canine
melanoma. Hum Gene Ther. (2015) 26:367–76. doi: 10.1089/hum.2014.130
- Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, et al. Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res. (2016) 22:4328–40.
doi: 10.1158/1078-0432.CCR-15-3026
- Cicchelero L, Denies S, Haers H, Vanderperren K, Stock E, Van Brantegem
L, et al. Intratumoural interleukin 12 gene therapy stimulates the immune system and decreases angiogenesis in dogs with spontaneous cancer. Vet Comp Oncol. (2017) 15:1187–205. doi: 10.1111/vco.12255
- Paoloni M, Mazcko C, Selting K, Lana S, Barber L, Phillips J, et al. Defining the pharmacodynamic profile and therapeutic index of NHS-IL12 immunocytokine in dogs with malignant melanoma. PLoS ONE. (2015) 10:e0129954. doi: 10.1371/journal.pone.0129954
- London CA, Gardner HL, Rippy S, Post G, La Perle K, Crew L, et al. KTN0158, a humanized anti-KIT monoclonal antibody, demonstrates biologic activity against both normal and malignant canine mast cells. Clin Cancer Res. (2017) 23:2565–74. doi: 10.1158/1078-0432.CCR-16-2152
- Maekawa N, Konnai S, Takagi S, Kagawa Y, Okagawa T, Nishimori A, et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci Rep. (2017) 7:8951. doi: 10.1038/s41598-017-09444-2
- Hogge GS, Burkholder JK, Culp J, Albertini MR, Dubielzig RR, Keller ET, et al. Development of human granulocyte-macrophage colony- stimulating factor-transfected tumor cell vaccines for the treatment of spontaneous canine cancer. Hum Gene Ther. (1998) 9:1851–61. doi: 10.1089/hum.1998.9.13-1851
- Turek MM, Thamm DH, Mitzey A, Kurzman ID, Huelsmeyer MK, Dubielzig RR, et al. Human granulocyte-macrophage colony-stimulating factor DNA cationic-lipid complexed autologous tumour cell vaccination in the treatment of canine B-cell multicentric lymphoma. Vet Comp Oncol. (2007) 5:219–31. doi: 10.1111/j.1476-5829.2007.00128.x
- Bird RC, Deinnocentes P, Church Bird AE, Van Ginkel FW, Lindquist J, Smith BF. An autologous dendritic cell canine mammary tumor hybrid-cell fusion vaccine. Cancer Immunol Immunother. (2011) 60:87–97. doi: 10.1007/s00262-010-0921-2
- Sorenmo KU, Krick E, Coughlin CM, Overley B, Gregor TP, Vonderheide RH, et al. CD40-activated B cell cancer vaccine improves second clinical remission and survival in privately owned dogs with non-Hodgkin’s lymphoma. PLoS ONE. (2011) 6:e24167. doi: 10.1371/journal.pone.0024167
- Marconato L, Frayssinet P, Rouquet N, Comazzi S, Leone VF, Laganga P, et al. Randomized, placebo-controlled, double-blinded chemoimmunotherapy clinical trial in a pet dog model of diffuse large B-cell lymphoma. Clin Cancer Res. (2014) 20:668–77. doi: 10.1158/1078-0432.CCR-13-2283
- U’Ren LW, Biller BJ, Elmslie RE, Thamm DH, Dow SW. Evaluation of a novel tumor vaccine in dogs with hemangiosarcoma. J Vet Intern Med. (2007) 21:113–20. doi: 10.1111/j.1939-1676.2007.tb02936.x
- Bergman PJ, Mcknight J, Novosad A, Charney S, Farrelly J, Craft D, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. (2003) 9:1284–90.
- Bergman PJ, Camps-Palau MA, Mcknight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for
canine malignant melanoma at the Animal Medical Center. Vaccine. (2006)
24:4582–5. doi: 10.1016/j.vaccine.2005.08.027
122. Kamstock D, Elmslie R, Thamm D, Dow S. Evaluation of a xenogeneic VEGF
vaccine in dogs with soft tissue sarcoma. Cancer Immunol Immunother.
(2007) 56:1299–309. doi: 10.1007/s00262-007-0282-7
123. Gavazza A, Lubas G, Fridman A, Peruzzi D, Impellizeri JA, Luberto L,
et al. Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Hum Gene Ther. (2013) 24:728–38. doi: 10.1089/hum.2013.112
124. Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier- Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. (2016) 22:4380–90. doi: 10.1158/1078-0432.CCR-16-0088
125. Mie K, Shimada T, Akiyoshi H, Hayashi A, Ohashi F. Change in peripheral blood lymphocyte count in dogs following adoptive immunotherapy using lymphokine-activated T killer cells combined with palliative tumor resection. Vet Immunol Immunopathol. (2016) 177:58–63. doi: 10.1016/j.vetimm.2016.06.007
126. O’Connor CM, Sheppard S, Hartline CA, Huls H, Johnson M, Palla SL, et al. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Sci Rep. (2012) 2:249. doi: 10.1038/srep00249
127. Hwang CC, Igase M, Sakurai M, Haraguchi T, Tani K, Itamoto K, et al. Oncolytic reovirus therapy: pilot study in dogs with spontaneously occurring tumours. Vet Comp Oncol. (2018) 16:229–38. doi: 10.1111/vco.12361
128. Naik S, Galyon GD, Jenks NJ, Steele MB, Miller AC, Allstadt SD, et al. Comparative oncology evaluation of intravenous recombinant oncolytic vesicular stomatitis virus therapy in spontaneous canine cancer. Mol Cancer Ther. (2018) 17:316–26. doi: 10.1158/1535-7163.MCT- 17-0432
129. Panjwani MK, Smith JB, Schutsky K, Gnanandarajah J, O’Connor CM, Powell DJ, et al. Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor T cells in dogs with spontaneous B Cell lymphoma. Mol Ther. (2016) 24:1602–14. doi: 10.1038/mt.2016.146
130. Walsh P, Gonzalez R, Dow S, Elmslie R, Potter T, Glode LM, et al. A phase I study using direct combination DNA injections for the immunotherapy of metastatic melanoma. Univ Colorado Cancer Center Clinical Trial Hum Gene Ther. (2000) 11:1355–68. doi: 10.1089/104303400500 32447
131. Yuan J, Ku GY, Adamow M, Mu Z, Tandon S, Hannaman D, et al. Immunologic responses to xenogeneic tyrosinase DNA vaccine administered by electroporation in patients with malignant melanoma. J Immunother Cancer. (2013) 1:20. doi: 10.1186/2051-1426-1-20
Conflict of Interest: The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Copyright © 2019 Thamm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.