Yomogi Satoa, Mohamed Elbadawya,b,*, Kazuhiko Suzukic, Ryouichi Tsunedomid,
Hiroaki Nagano d, Yusuke Ishihara a, Haru Yamamoto a, Daigo Azakami e, Tsuyoshi Uchide f, Rina Nabeta f, Ryuji Fukushima g, Amira Abugomaa a, h, Masahiro Kaneda i,
Hideyuki Yamawaki j, Yuta Shinohara k, Tatsuya Usui a, **, Kazuaki Sasaki a
a Laboratory of Veterinary Pharmacology, Department of Veterinary Medicine, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan
b Department of Pharmacology, Faculty of Veterinary Medicine, Benha University, 13736, Moshtohor, Toukh, Elqaliobiya, Egypt
c Laboratory of Veterinary Toxicology, Department of Veterinary Medicine, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan
d Department of Gastroenterological, Breast and Endocrine Surgery, Yamaguchi University Graduate School of Medicine, 1-1-1 Minami-Kogushi, Ube, Yamaguchi 755- 8505, Japan
e Laboratory of Veterinary Clinical Oncology, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan f Laboratory of Veterinary Molecular Pathology and Therapeutics, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan
g Animal Medical Emergency Center, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 2-24-16 Nakamachi, Koganei, Tokyo 184-8588, Japan h Faculty of Veterinary Medicine, Mansoura University, 35516 Mansoura, Egypt
i Laboratory of Veterinary Anatomy, Department of Veterinary Medicine, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan
j Laboratory of Veterinary Pharmacology, School of Veterinary Medicine, Kitasato University, 35-1, Higashi 23 ban-cho, Towada, Aomori 034-8628, Japan k Pet Health & Food Division, Iskara Industry CO., LTD, 1-14-2, Nihonbashi, Chuo-ku, Tokyo, 103-0027, Japan
ARTICLEINFO
Keywords:
Pleural mesothelioma Dogs
Organoids
RNA-seq
Cell adhesion EMT
ABSTRACT
Canine malignant mesothelioma (cMM) is a rare and drug-resistant malignant tumor. Due to few patients and experimental models, there have not been enough studies to demonstrate the pathogenesis of the disease and novel effective treatment for cMM. Since cMM resembles human MM (hMM) in histopathological characteristics, it is also considered a promising research model of hMM. Compared with conventional 2-dimensional (2D) culture methods, 3-dimensional (3D) organoid culture can recapitulate the properties of original tumor tissues. However, cMM organoids have never been developed. In the present study, we for the first time generated cMM organoids using the pleural effusion samples. Organoids from individual MM dogs were successfully generated. They exhibited the characteristics of MM and expressed mesothelial cell markers, such as WT-1 and mesothelin. The sensitivity to anti-cancer drugs was different in each strain of cMM organoids. RNA sequencing analysis showed cell adhesion molecule pathways were specifically upregulated in cMM organoids compared with their corresponding 2D cultured cells. Among these genes, the expression level of E-cadherin was drastically higher in the organoids than that in the 2D cells. In conclusion, our established cMM organoids might become a new experimental tool to provide new insights into canine and human MM therapy.
1. Introduction
Mesothelium is the serous epithelium composed of mesothelial cells
which have both epithelial and mesenchymal cell properties, covering the body cavities and the surface of organs [1].
Mesothelial cells, which normally exist in a single sheet-like layer, become multilayered and free
* Corresponding author at: Laboratory of Veterinary Pharmacology, Department of Veterinary Medicine, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan.
** Corresponding author.
E-mail addresses: Mohamed.elbadawy@fvtm.bu.edu.eg (M. Elbadawy), fu7085@go.tuat.ac.jp (T. Usui).
https://doi.org/10.1016/j.biopha.2023.114651
Received 28 December 2022; Received in revised form 21 March 2023; Accepted 31 March 2023
Available online 6 April 2023
0753-3322/© 2023 The Authors. Published by Elsevier Masson SAS. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
in the body cavity effusions when proliferation is activated by inflam- mation or tumorigenesis [2]. Malignant mesotheliomas (MM) are known to occur in a variety of serous cavities in several mammalian species such as humans, dogs, cats, cows, and mice. In humans, pleural meso- thelioma is the most common and has been specifically linked to asbestos exposure [3]. However, the exact cause of the disease in dogs is unknown. According to the WHO classification [4,5], MM can be his- tologically classified into three major subtypes (epithelioid, sarcoma- toid, and biphasic). Dogs are considered a good model for new drugs and therapeutic methods since they present spontaneous diseases like humans [6–10].
Canine MM (cMM) is rare and more aggressive with a poor prognosis [11]. It arises mainly from the mesothelial lining of the pleura, perito- neum, and pericardium or rarely from the tunica vaginalis and testis [11]. Although it affects middle-aged to elderly dogs, there have also been reports about younger-onset cases [12–14]. The main clinical symptoms include tachypnoea and dyspnoea due to pleural effusion and ascites [11].
There is currently no optimal treatment for cMM. It is considered an intractable tumor due to poor response or treatment resistance [11]. Treatments include intracavitary or intravenous platinum-based chemotherapy, 5-FU, and carboplatin, alone or with mitoxantrone [11, 16–18]. The treatments decreased effusion volume and prolonged the mean survival time to some extent [11]. To improve the treatment strategy of cMM, developing new preclinical models are necessary. In humans, mice models [19,20], cell lines [21,22], spheroids [23–25], and organoids [26,27] have been used for MM research. On the other hand, due to the small number of cases and the relatively rapid progression of the disease, most of the studies and reports on cMM have been based on clinical studies [11,15,28], and few MM cell lines have been established in dogs [17,18,28–30]. Therefore, a new experimental 3D model needs to be established to advance research on novel therapeutic agents and tumor dynamics of cMM.
Recently, the organoid culture method has been applied in the field of regenerative medicine [31], precision medicine [32], modeling dis- eases [33,34], toxicologic pathology [35], translational research [36], and tumor research [37–39]. Organoids are 3-dimensional (3D) cultured
cells that are rich in self-renewal ability. They are maintained in a state more similar to that of a living body [40,41]. Thus, it is considered a precious model for studying the biology of cMM and for improving its treatment strategies. Based on our knowledge, organoid culture methods for cMM have not been established. Therefore, in the present study, we aimed to establish a method for culturing patient-derived cMM orga- noids and investigated the differences in molecular characteristics compared with 2D cells.
2. Materials and methods
2.1. Materials
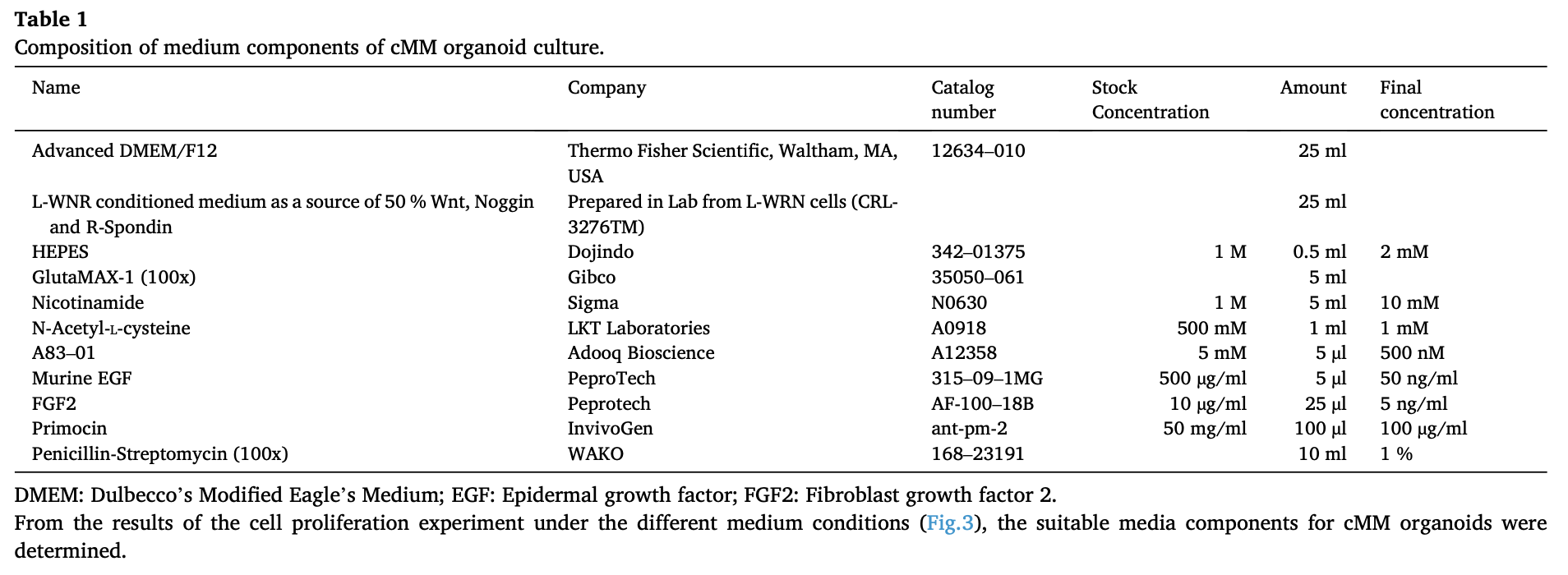
To generate cMM organoids, cells of pleural effusion were embedded in Matrigel (BD Bioscience, San Jose, CA, USA) and cultured with a modified stem cell-enhancement medium as described before [8,33,41]. The culture medium was advanced Dulbecco’s Modified Eagle’s Medium (Ad. DMEM, Thermo Fisher Scientific, Waltham, MA, USA). Stemness supplements and growth factors were as follows: 50 % Wnt (W)-, Noggin (N)-, and R-Spondin (R)-conditioned medium; GlutaMax; 100 μg/ml Primocin (Thermo Fisher Scientific, Waltham, MA, USA); 1 mM N-Acetyl-L-cysteine; 10 mM nicotinamide (Sigma-Aldrich, St. Louis, MO, USA); and 500 nM A83–01 (Adooq Bioscience, Irvine, CA, USA), 50 ng/ml epidermal growth factor (EGF) (Pepro Tech, Cranbury, NL, USA); 5 ng/ml fibroblast growth factor (FGF)− 2 (FGF2) (R&D Systems, Inc. Minneapolis, MN, USA); 5 ng/ml FGF7; 25 ng/ml FGF10 (Pepro Tech); and 100 ng/ml insulin-like growth factor (IGF) (Novus Biologicals, Centennial, CO, USA). Further details are shown in Table 1. Anti-cancer drugs were as follows: carboplatin (FUJIFILM WAKO Pure Chemical Corporation, Osaka, Japan), doxorubicin, cisplatin, and gemcitabine (Cayman, Ann Arbor, MI, USA). Antibody sources were as follows: anti-AE1/AE3 (Novus Biologicals); anti-mesothelin (Abcam, Cambridge, MA, USA); anti-Wilms’ tumor-1 (WT-1, Dako-Agilent, Technologies Inc., Santa Clara, CA, USA); anti-vimentin (Santa Cruz Biotechnology, Dallas, TX, USA); and anti-E-cadherin (BD Bioscience). The secondary antibody was EnVision+Dual Link System-HRP (Dako-Agilent).
2.2. cMM sample information
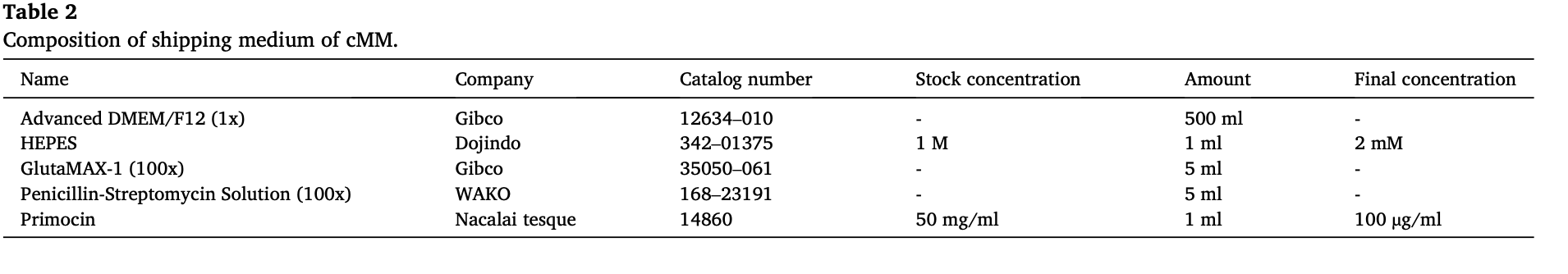
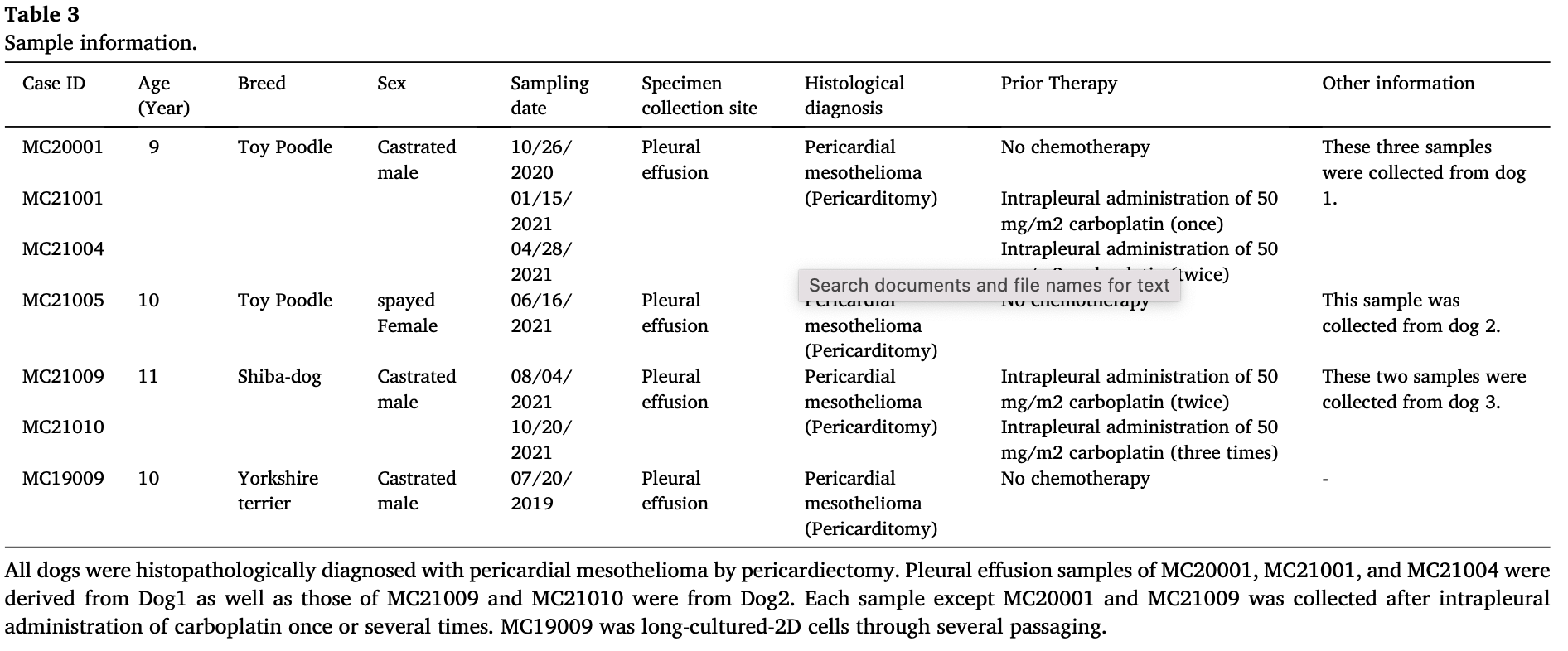
The pleural effusion samples were collected from three dogs that were histopathologically diagnosed with pericardial mesothelioma by pericardiectomy. All samples were collected (2019–2021) from Animal Medical Center, Tokyo University of Agriculture and Technology, Fuchu, Tokyo, Japan, and transferred immediately to our laboratory in a shipping medium (Table 2). Information about dogs and their samples in the present study is listed in Table 3. For the present study, written informed consents were obtained from all dogsʼ owners, and the ex- periments were carried out with direction from the Institute Animal Care and Use Committee of Tokyo University of Agriculture and Technology approval (Approval number: 0016012).
2.3. Generation of cMM organoids
The pleural fluid samples from dogs diagnosed with cMM were centrifuged at 600 g for 3 min, and the pellets were collected after being washed three times with a cold phosphate-buffered saline (PBS). The cell pellets were mixed with Matrigel (40 μl/well) on ice and seeded on 24- well plates. After solidifying the gel at 37 °C for 30 min, the culture medium (Table 1) was added and changed triple per week. After confluent growth, cMM organoids were passaged into new wells at a ratio of 1:3–4 as described before [6].
2.4. Generation of cMM 2D cells
The cMM cell pellets were collected from the pleural effusion sam- ples, incubated with 1x RBC lysis buffer (pluriSelect, Leipzig, Germany) for 3 min at room temperature (RT), centrifuged at 200 g for 3 min, and washed three times with PBS. Cells were then mixed with Roswell Park Memorial Institute (RPMI)-1640 (Sigma-Aldrich) supplemented with 10 % fetal bovine serum (FBS, Sigma Aldrich), and 1 % penicillin- streptomycin (WAKO), 1 % L-glutamine, 50 ng/ml EGF, 0.1 % hydro- cortisone (WAKO) and 0.2 % heparin (Sigma Aldrich), and cultured in a 6 cm dish. The medium was changed three times weekly and passaged at 80 % confluent condition. The cultured cells were used for the subse- quent analysis.
2.5. Appropriate medium component assay for cMM organoids
After the cMM organoids grew enough, they were treated with 5 mM EDTA/PBS for 90 min on ice and trypsinized for 5 min at 37 ◦C using TrypLE (Thermo Fisher Scientific Inc.). Subsequently, the organoids
were vigorously pipetted and passed through a 70 μm cell strainer (Falcon, Cary, NC, USA). The obtained cell solution was neutralized with FBS and centrifuged at 600 g for 3 min. The cell pellets were then collected, mixed with Matrigel on ice, and seeded in 96-well culture plates. After the gel solidify in a CO2 incubator for 20 min, the culture media of different compositions were added. The used culture media were as follows: (1) Control medium contained Advanced DMEM; Glu- taMax; 100 μg/ml Primocin; 1 mM N-Acetyl-L-cysteine; 10 mM nico- tinamide; and 500 nM A83–01. (2) WNR medium was composed of the control medium with 50 % Wnt (W), Noggin (N), and R-Spondin (R) conditioned medium. Media of (3) to (7) contained control medium with 50 ng/ml EGF, 5 ng/ml FGF2, 5 ng/ml FGF7, 25 ng/ml FGF10, and 100 ng/ml IGF, respectively. The media were changed three days later, and cell proliferation rates were determined after 7 days using a PrestoBlue assay kit (Thermo Fisher Scientific) and a microplate reader (TECAN, Seestrasse, Switzerland) at an emission wavelength of 585 nm. Data were analyzed and graphed using SigmaPlot software (V 14.5, Systat Software, Inc. San Jose, CA. USA).
2.6. Hematoxylin and eosin (H&E) staining of cMM organoids
H&E staining of organoids was carried out as described before [6,8]. After being fixed with 4 % paraformaldehyde (PFA) at 4 °C overnight, each organoid sample was dehydrated with ethanol and xylene and then embedded in paraffin for microtome slicing into 4-μm-thick sections and mounted onto MS-coated glass slides. Thereafter, sections were depar- affinized followed by H&E staining according to the standard proced- ures. The images were captured by a light microscope (BX-52; Olympus, Tokyo, Japan).
2.7. Immunohistochemical (IHC) staining of cMM organoids
The IHC staining of cMM organoids was performed as described before [6,8]. After deparaffinization of cMM organoid sections with xylene and ethanol, the antigen was retrieved in 10 mM citrate buffer with heating at 121 °C for 5 min, followed by inactivating the endoge- nous peroxidase activity by treating the sections with 1 % peroxidase for 30 min. Subsequently, after being blocked with 10 % normal goat serum (NGS) / Tris-buffered saline (TBS) for 30 min, the samples were incu- bated at 4 °C overnight with primary antibodies (anti-cytokeratin AE1/AE3; 1:100, anti-mesothelin; 1:100, anti-WT1; ready to use, anti-vimentin; 1:200, and anti-E-cadherin; 1:200). The sections were then washed three times with PBS and incubated with the secondary antibody (EnVision+ Dual Link System-HRP) and visualized by using
the DAB solution (Nacalai Tesque, Tokyo, Japan). Nuclei were coun- terstained with Mayer’s hematoxylin. All images were obtained using a light microscope (BX-43; Olympus, Tokyo, Japan).
2.8. Immunocytochemistry (ICC) of cMM 2D cells
The ICC staining of cMM 2D cells was performed as described before
[42]. The cultured cMM 2D cells were washed with PBS and fixed with methanolcooledat− 20°Cfor5minbeforeinactivatingtheendogenous peroxidase activity by treating them with 1 % peroxidase for 20 min. Thereafter, the samples were blocked with 10 % NGS/TBS for 30 min followed by incubation with primary antibodies (anti-cytokeratin AE1/AE3; 1:100, anti-mesothelin; 1:100, anti-WT; ready to use, anti-vimentin; 1:200, and anti-E-cadherin; 1:200). Subsequently, 2D cell samples were incubated with the secondary antibody (EnVision+ Dual Link System-HRP, DAKO) and visualized by using the DAB solution (Nacalai Tesque). Nuclei were counterstained with Mayer’s hematoxy- lin. All images were obtained using a light microscope (BX-43).
2.9. Transmission electron micrograph (TEM) of cMM organoids
The TEM procedure was carried out as mentioned previously [34]. Briefly, cMM organoids were collected into 15-ml tubes and fixed overnight with 2.5 % glutaraldehyde (pH 7.4). The organoids were then washed once with 0.1 M cacodylate (pH 7.4) and incubated for 2 h at 4 °C in a solution of 2 % osmium tetroxide and 1.5 % K4Fe(CN)6 in 0.1 M sodium cacodylate (pH 7.4). Thereafter, the organoids were washed with PBS, dehydrated in graded ethanol solutions (50 %, 70 %, 80 %, 90 %, 95 %, and 99.5 up to 100 %), and embedded in Epon. Ultrathin sections (70–110 nm) were sliced with a diamond knife using a Leica UC7 ultramicrotome and loaded onto 50-mesh copper grids coated by form bar and carbon film. The sections were post-stained with uranyl acetate for 15 min at RT and lead citrate. TEM images were prepared using a TEM (H-7500, Hitachi, Tokyo, Japan) with a TEM digital camera (NanoSprint500, Hitachi).
2.10. Drug treatment and cell viability assay
Cell viability of cMM organoids and 2D cells was carried out as described before [6,39]. The appropriate concentrations for carboplatin, doxorubicin, cisplatin, and gemcitabine were determined based on their blood therapeutic levels in the clinical and pharmacokinetics data [11, 43–45]. Both cMM organoids and 2D cells were washed by PBS and treated with 5 mM EDTA/PBS, and then were trypsinized using TrypLE. For organoids, after being trypsinized, the organoid cells were passed through a cell strainer (70 μm). Then, both organoids and 2D cells were neutralized by FBS and counted. Approximately 2 × 103 cells of orga- noid were embedded in 10 μl Matrigel or 2D cells with culture media in 96-well culture plates and incubated for 24 h. Later, the cells were treated with DMSO or each drug as follows: carboplatin (0.1, 1, 10, and 100 μg/ml), doxorubicin (0.1, 1, 10, and 100 μM), cisplatin (0.1, 1, 10, and 100 μM) or gemcitabine (0.1, 1, 10, and 100 μM) for 72 h. Cell viability was analyzed using the PrestoBlue kit (Thermo Fisher Scientific Inc.) by a microplate reader (TECAN) at an emission wavelength of 585 nm. Bright-field images were captured using the light microscope (BX-52) and the readings of the microplate reader were analyzed and graphed using SigmaPlot software.
2.11. RNA sequence analysis
The RNA sequencing (RNA-Seq) analysis was performed as described before [46]. Total RNA was extracted from cMM organoids and 2D cells using the NucleoSpin kit (TaKaRa Bio Inc.) following the manufacturer’s instructions. The total extracted RNA (10 ng) for each sample was used to generate the libraries for sequencing. A Ribo-Zero Human Kit (Illu- mina, San Diego, CA, USA) and a TruSeq Stranded Total RNA Library
Biomedicine & Pharmacotherapy 162 (2023) 114651
Prep Kit (Illumina) were used to equip the library, followed by sequencing (7.5 million paried-end reads) on an Illumina NovaSeq in- strument. Initial quality control of RNA-seq data (FASTQ) for each sample was carried out by cutadapt (version 1.8.3) and cmpfastq_pe.pl software. Reads were mapped to the reference genome (CanFam 3.1) using the STAR software (version 2.5.1b). Principle components analysis (PCA) was performed to display differences between samples. Read counts were normalized using the trimmed mean of the M value method. To recognize the upregulated genes in samples compared to control ones, the mean values were calculated and shown as a fold increase relative to those of the control value. Gene set enrichment analysis was performed with a java command line program, GSEA (version 4.1.0), and Molecular Signatures Database v7.4 [47].
2.12. Quantitative real-time polymerase chain reaction
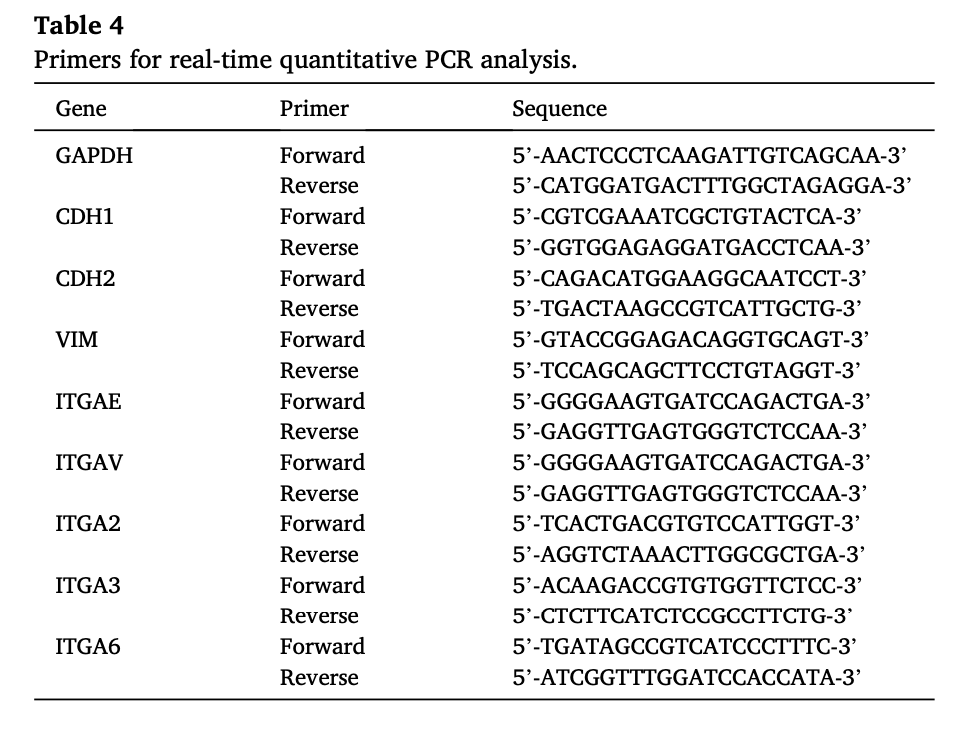
Total RNA was extracted from cMM organoid samples and cMM 2D cell samples using the NucleoSpin kit (Takara Bio Inc, Japan) according to the manufacturer’s instructions. First-strand cDNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen Hilden, Germany). Quantitative real-time PCR was performed using a QuantiTect SYBR I Kit (Qiagen) and a StepOnePlus Real-Time PCR System (Applied Bio- systems, Waltham, MA, USA). The 2-ΔΔCq method and cycle threshold (Ct) values obtained in quantification were used to calculate the fold changes in mRNA abundance. Specific primer designs are shown in Table 4.
2.13. Statistical analysis
Data are shown as mean ± SEM. Statistical evaluation was per- formed by SigmaPlot software using one-way analysis of variance (ANOVA) followed by Bonferroni’s test. When P values (two-tailed) are < 0.05, the data were considered statistically significant.
3. Results
3.1. Generation of cMM organoids from pleural effusion samples of cMM- diseased dogs
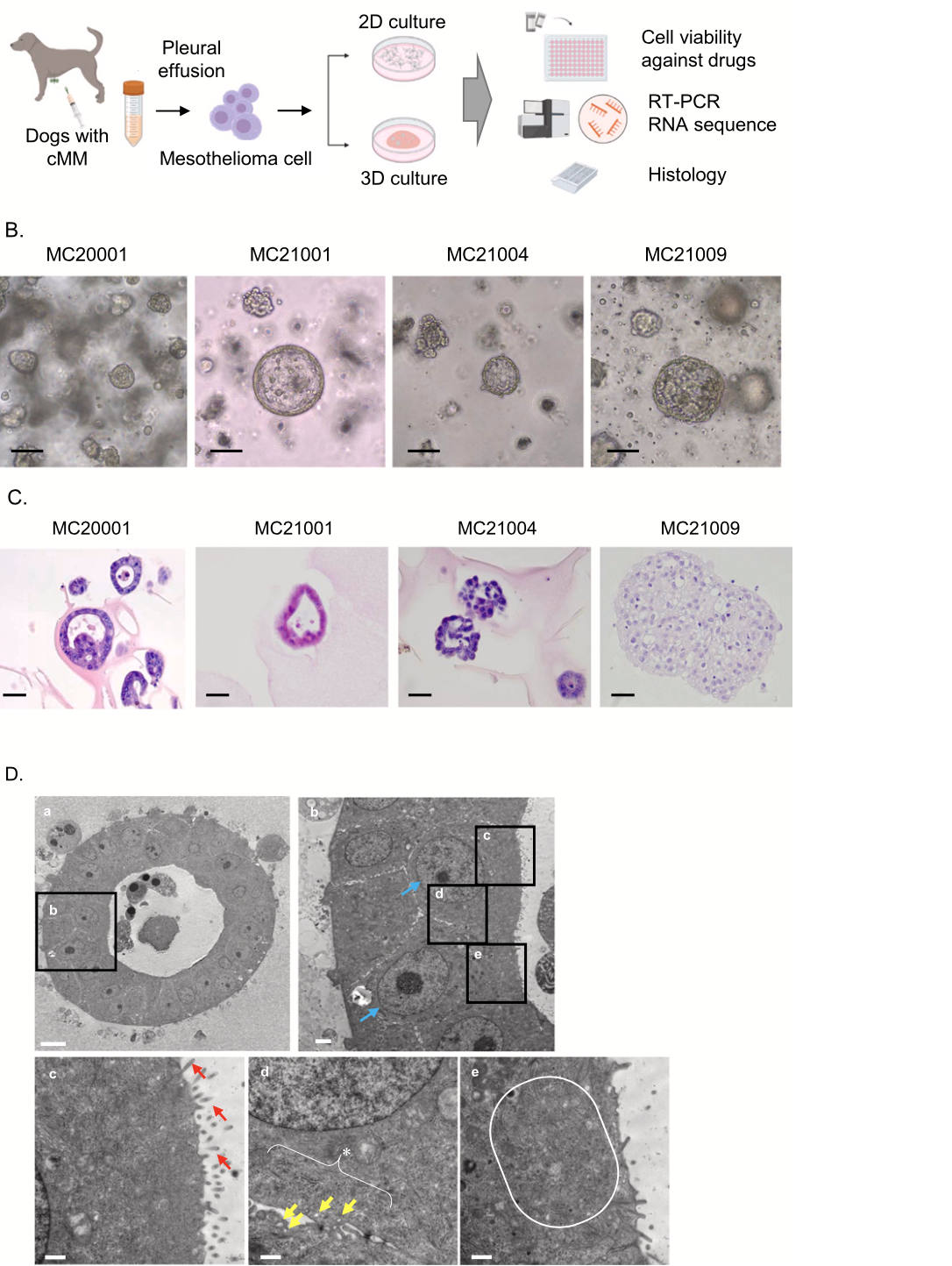
Mesothelial cells were collected from pleural effusion of dogs with cMM, whose information was shown in Table 3. The collected cells were seeded into 6 cm dishes as 2D culture and 24-well plates with Matrigel as 3D culture (Fig. 1A). All samples from pleural effusion successfully formed spherical organoids after culturing for 3–7 days (Fig. 1B) and continued to grow after several passages. The cMM organoids showed not just spherical but luminal-like formations (Fig. 1B, C). Although
Fig. 1. Generation of canine malignant mesothelioma (cMM) organoids. Schematic experimental design of a procedure of generation and comparative analysis of cMM organoids and 2D cultured cells (A). Mesothelioma cells were collected from the pleural effusion of dogs with malignant mesothelioma. The cells were seeded into 6 cm dishes as 2-dimensional (2D) culture and 24-well plates with Matrigel as 3-dimensional (3D) culture. After several times of passaging, the cells were investigated for cell viability, gene expression, and histology. Representative images for bright-field microscopy (B) and hematoxylin and eosin (H&E) staining (C) of cMM organoids. Scale bar: 100 μm (B) and 25 μm (C). Transmission electron micrograph (TEM) of cMM organoids (D). One whole organoid image (a) and its enlarged portions (b, c, d, e) were shown. Red arrows show microvilli (c). Yellow arrows show drinking action cells (d). Asterisk marks show glycogen granules (d). The white circle shows microfilaments (e). Scale bar: 10 μm (a), 2 μm (b), and 600 nm (c, d, e).
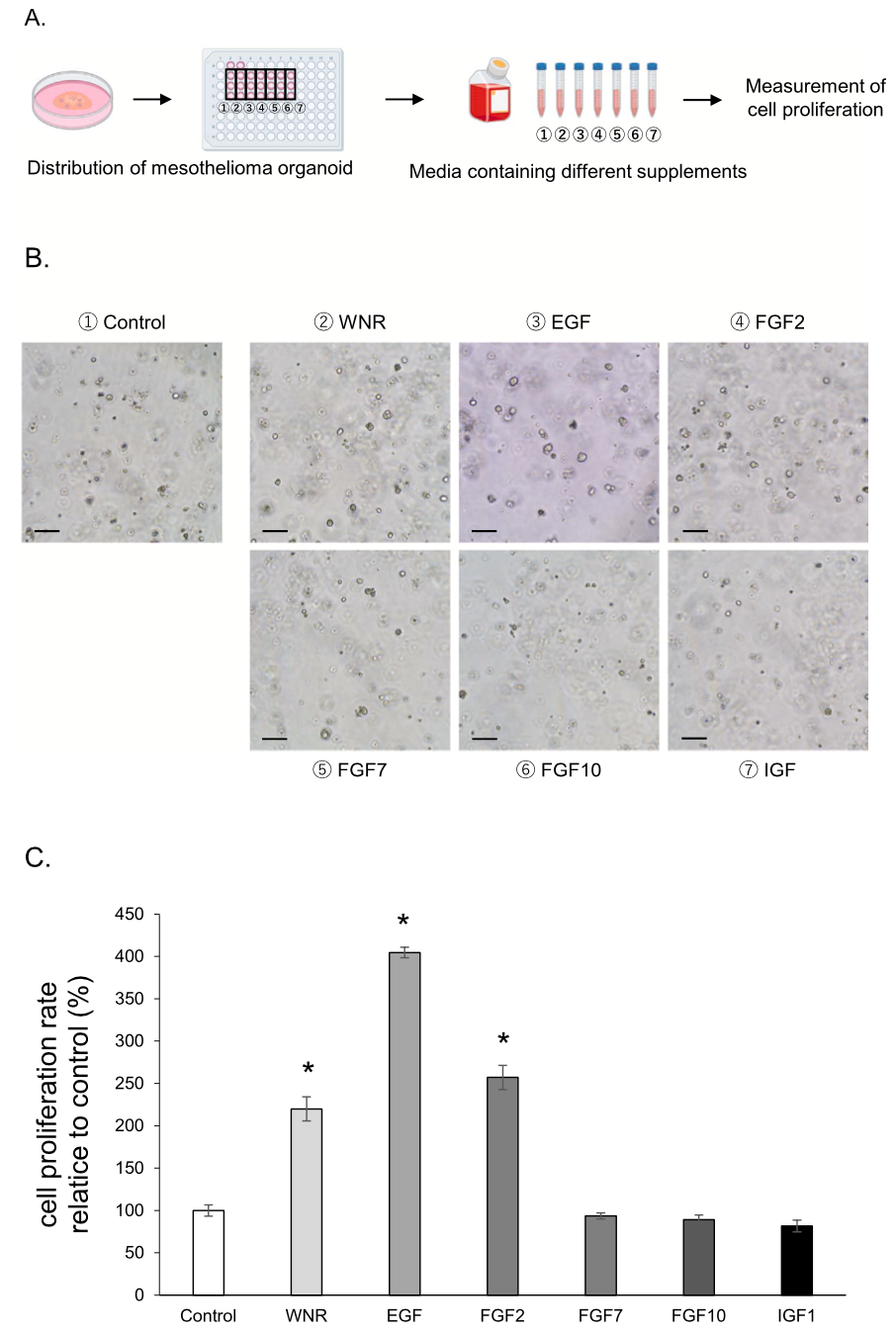
Fig. 2. Assessment of appropriate medium components for cMM organoids. Experimental schema of assessment of appropriate medium components of cMM organoids (A). Phase- contrast images of the growth of cMM organo- ids (MC20001) cultured in base medium alone (Cont) or with different culture supplements after seeding the same number of cells. WNR indicates Wnt 3a, Noggin, and R-spondin. Scale bar: 100μm (B). Cell proliferation ratio of organoids in each culture media (C, n = 3). The results were shown as a fold increase relative to control and expressed as mean ± S.E.M. *P < 0.05 vs. control.
most cMM organoids gradually decreased their growth speed, the growth finally stopped after five to six passages. TEM images of cMM organoids (MC20001) showed distinct nucleoli and euchromatin dis- tribution (Fig. 1D. a, b), well-developed microvilli (Fig.1D. c), numerous drinking action cells, and glycogen granules (Fig.1D. d), randomly ar- ranged microfilaments (Fig.1D. e). Since these observations corre- sponded with the characteristics of MM, it was implied that our established cMM organoids reflect the properties of malignant mesothelioma.
On the other hand, cMM 2D cultured cells were also generated from pleural effusion samples and most samples except MC21001 showed
mesenchymal-like morphology (Supplementary Fig. 1A). MC21001 2D cells exhibited mesenchymal-like morphology at first, changing their formation to epithelial-like cells through passaging (Supplementary Fig. 1A). Unlike cMM organoids, cMM 2D cells kept growing after more than 10 times passaging.
3.2. Assessment of appropriate medium components for cMM organoids
Although it was reported that RPMI and DMEM media were used for the 2D culture of hMM cells [45,48], suitable medium conditions for culturing MM organoids have not been reported. We, therefore,
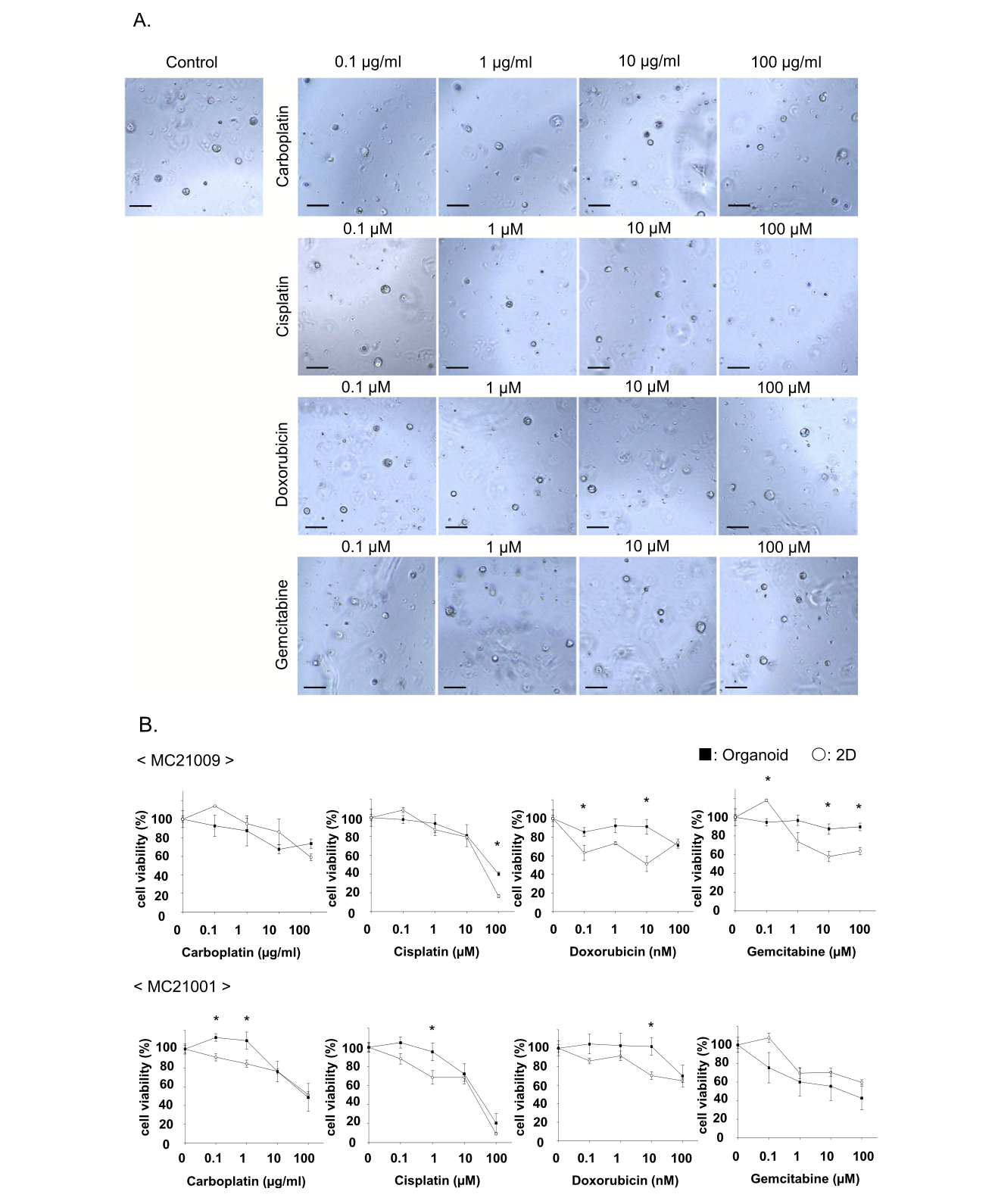
Fig. 4. Comparison of sensitivity to anti-cancer drugs between cMM organoids and 2D cells. After 2 strains of cMM organoids or 2D cells were seeded, they were treated with carboplatin (0.1–100 μg/ml), cisplatin (0.1–100 μM), doxorubicin (0.1–100 nM), or gemcitabine (0.1–100 μM) for 72 h. Representative phase contrast images of cMM organoids (MC21009) treated with carboplatin, cisplatin, doxorubicin, or gemcitabine were shown (A). Scale bar: 100 μm. Cell viability was assessed using a Prestoblue cell viability assay, and 100 % represents the cell viability of each control (n = 3) (B). Results were expressed as mean ± S.E.M. * P < 0.05 vs. organoid.
investigated the suitable medium components for cMM organoids. Approximately 2 × 103 cells of cMM organoids were seeded in a 96-well plate and their proliferation rate was assessed after culturing for 7 days by using a microplate reader (n = 3) (Fig. 2A). On day 7, the prolifer- ation rate was significantly higher when using the base media (Cont) with WNR, EGF, or FGF2 (Fig. 2B, C). FGF7, FGF10, and IGF had no significant effects compared with the control. Based on these data, we selected WNR, EGF, and FGF2 as the best components for cMM organoid culture (Fig. 2C, Table 1).
3.3. Expression of mesothelioma cell markers in cMM organoids
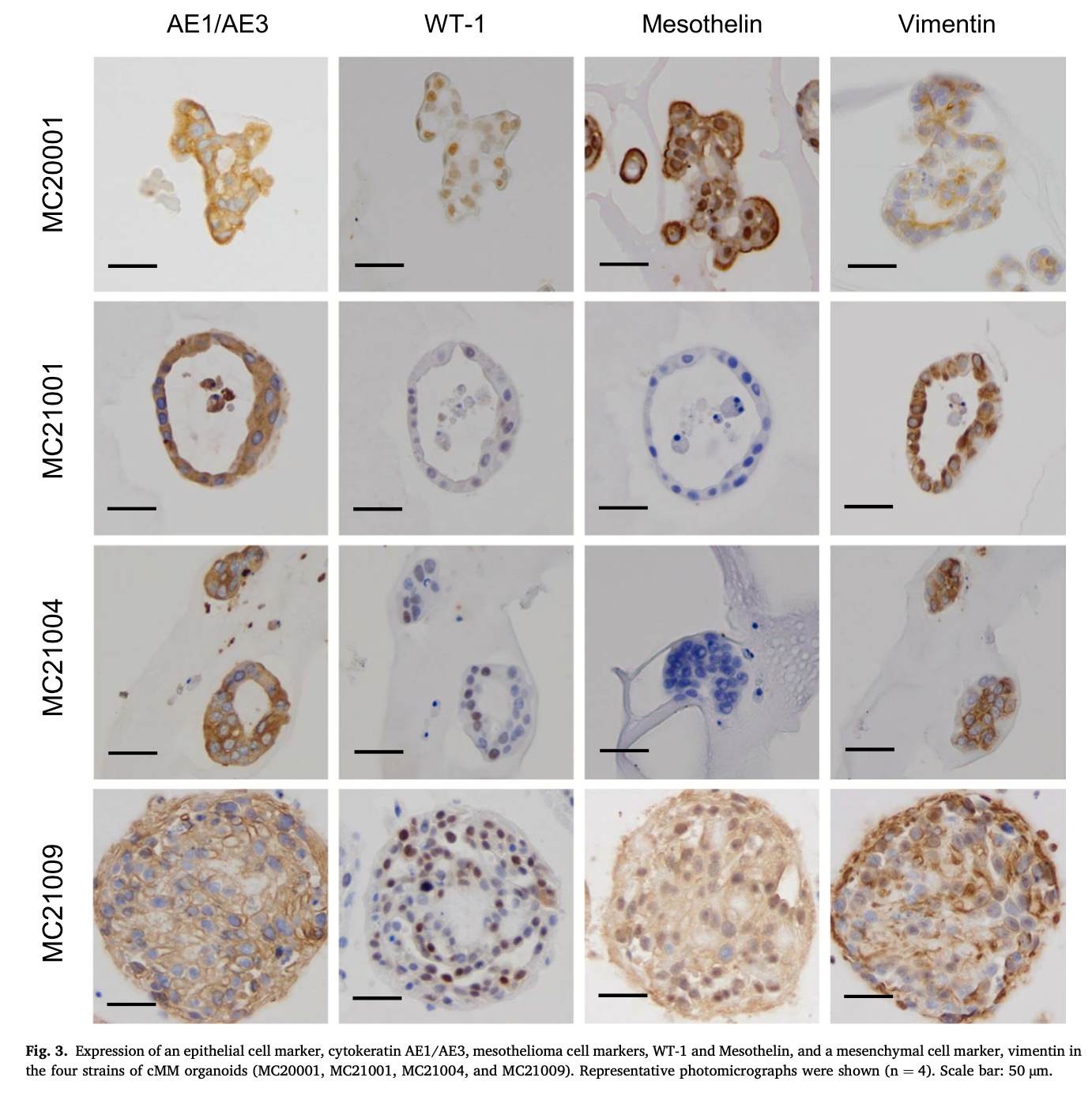
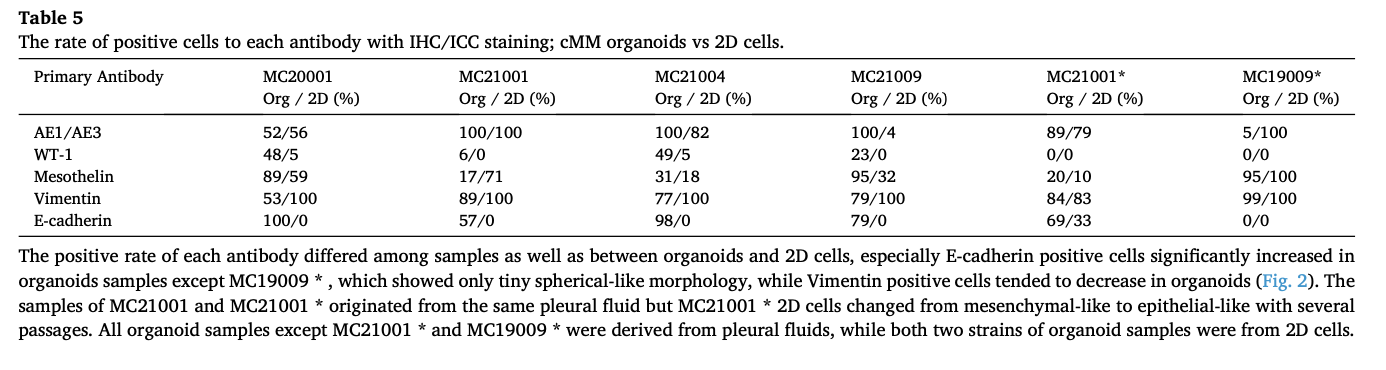
In the IHC staining experiment, expression of an epithelial cell marker, cytokeratin AE1/AE3, mesothelioma cell markers, WT-1 and Mesothelin, and a mesenchymal cell marker, vimentin was observed in each strain of cMM organoids (Fig. 3). The positive cells for cytokeratin AE1/AE3 and vimentin were higher than other two antibodies. In addition, the ratio of positive cells differed between each organoid sample and also between organoid and 2D cells (Table 5). The cMM 2D cells were also assessed by ICC staining for the same antibodies (Sup- plementary Fig. 1B). Although expression of cytokeratin AE1/AE3 and vimentin were also observed in each strain of 2D cells (Supplementary Fig. 1B), expression of WT-1 and Mesothelin was seldom observed in some strains.
3.4. Comparison of sensitivity to anti-cancer drugs between cMM organoids and 2D cells
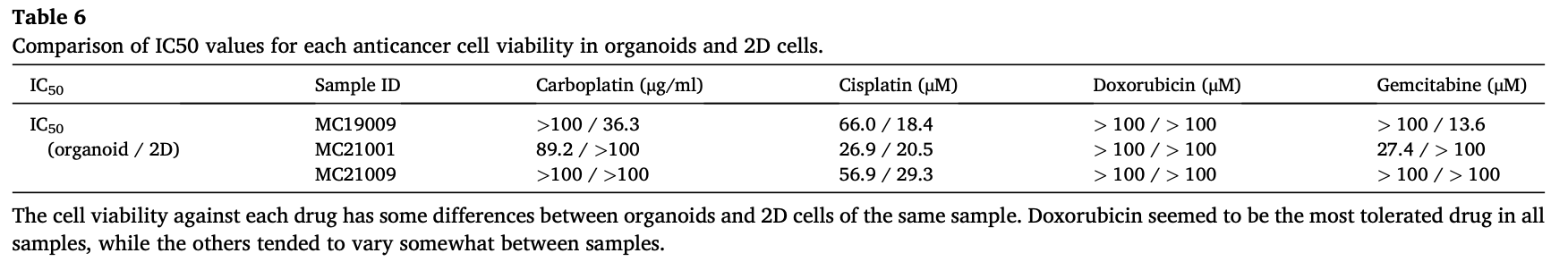
To prove the utility of cMM organoids as a preclinical model for checking the sensitivity to anti-cancer drugs, we examined the drug susceptivity of cMM by comparing organoids with 2D cells against four drugs (carboplatin, cisplatin, doxorubicin, and gemcitabine) as described previously (n = 3) [6,49,50]. As shown in the representative images of MC21009 organoids under the different drug conditions, treatment with a high concentration of cisplatin drastically decreased cell viability of MC21009 organoids, while treatment with carboplatin, doxorubicin, or gemcitabine partially decreased it (Fig. 4A, B). In MC21009 organoids, treatment with low concentrations of doxorubicin or high concentration of gemcitabine less decreased cell viability compared with 2D cells. Treatment with carboplatin or cisplatin showed similar inhibition of cell viability (Fig. 4B). In MC21001 organoids, treatment with low concentrations of carboplatin, cisplatin, or doxoru- bicin less decreased cell viability compared with 2D cells. On the other hand, treatment with the highest concentration of carboplatin, cisplatin, or doxorubicin showed similar inhibition of cell viability (Fig. 4B). The IC50 values for each anti-cancer drug in organoids and 2D cells were shown in Table 6.
3.5. Comparison of gene expression profile between cMM organoids and 2D cells
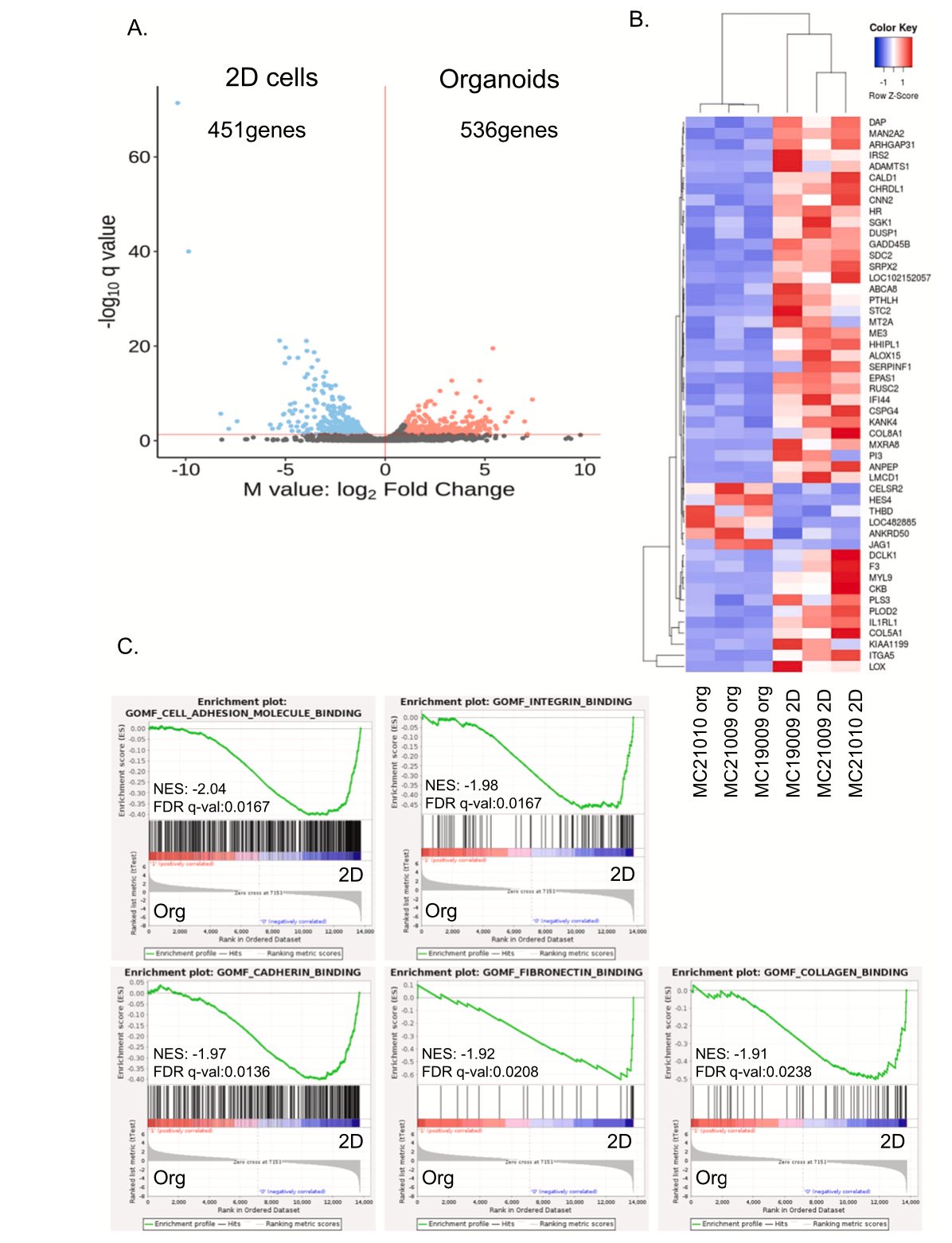
To investigate the differences in gene expression between cMM organoids and 2D cells, we analyzed the gene expression level of three strains of cMM organoids and 2D cells by RNA-seq. The expression level of 987 genes was significantly different between organoids and 2D cells. Among them, 536 genes were upregulated in organoids (Fig. 5 A). The
top 50 genes that were significantly upregulated in organoids or 2D cells were shown as a heatmap (Fig. 5B). Gene Set Enrichment Analysis (GSEA) showed cMM organoids were significantly enriched with cell binding-related signals, such as cell adhesion molecule binding, integrin binding, and cadherin binding pathways compared with 2D cells (Fig. 5 C). Interestingly, mesothelin mRNA expression tended to be higher in 3D organoids, while WT-1 mRNA expression differed among strains (Supplementary Fig. 2).
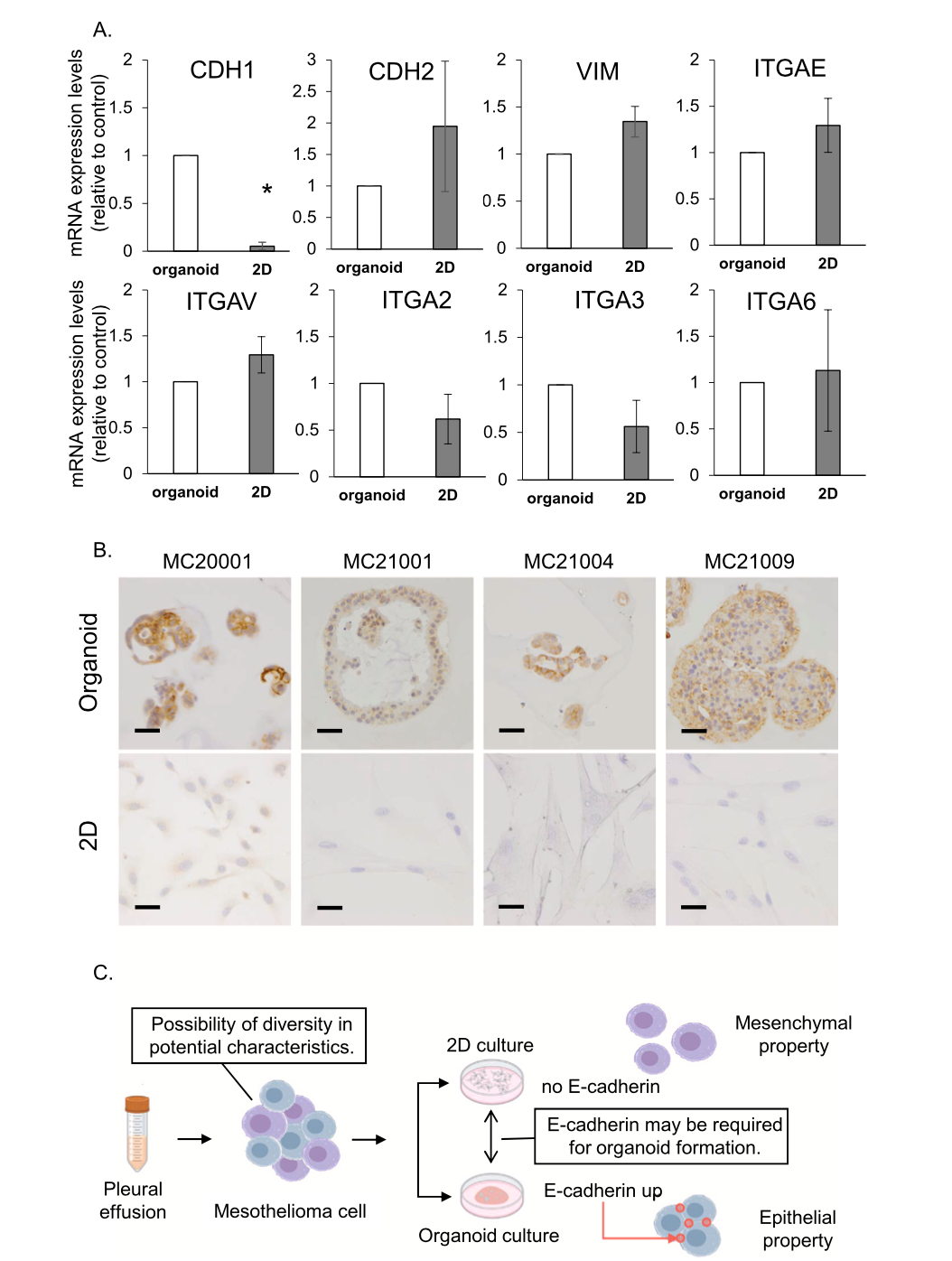
3.6. Cell-binding-related molecule expressions in cMM organoids and 2D cells
From the results of RNA-seq and GSEA, we evaluated the expression of some molecules associated with cell bindings by quantitative real- time PCR (n = 4). CDH1 (E-cadherin) is known as an epithelial marker expressed on the cell membrane and binding cell, while CDH2 (N-cad- herin) and Vimentin (VIM) are known to increase in mesenchymal cells or to be related to epithelial-mesenchymal transition (EMT). Integrin subunit alpha (ITGA) has a large subtype family such as ITGAE, ITGAV, and ITGA2, 3, 6, which are related to cell-cell adhesion and cancer in- vasion (Fig. 6A). CDH2 and VIM tended to decrease in organoids compared with 2D cells, while CDH1 significantly increased in orga- noids (Fig. 6A). To confirm the protein expression of E-cadherin, IHC or ICC staining experiment was performed. As with the results of quanti- tative real-time PCR, E-cadherin expression was highly detected in each strain of cMM organoids but not in 2D cells (Fig. 6B).
4. Discussion
In the current study, cMM organoids were successfully generated from pleural effusion, and specifically, the differences in characteristics related to cell adhesion were revealed by comparison with cMM 2D cells. The main findings of the current study are as follows: 1) The cMM organoids derived from pleural effusion showed a spherical or luminal morphology and expressed mesothelioma markers, mesothelial cell properties, and malignant features (Figs. 1 and 3), 2) EGF, FGF2, and WNR are necessary for the optimum growth of cMM organoids (Figs. 2 and 3) The cMM organoids showed more resistance against low con- centrations of each anti-cancer drug than 2D cells, while few differences were found between organoids and 2D cells under high concentrations of anti-cancer drugs (Fig. 4), 4) Compared with cMM 2D cells, cMM organoids from pleural effusion have different characteristics in cell adhesion molecules, such as E-cadherin (Figs. 5 and 6). Collectively, our data indicate that cMM organoids revealed different characteristics from their 2D cells and could be available as a novel experimental model for MM to provide new insights for MM treatment.
The histological classification of MM is widely common across mammals and is often divided into epithelioid (mainly composed of epithelial-shaped cells), sarcomatoid (mainly composed of spindle- shaped cells), or biphasic (composed of both types of cells), similar to human MM (hMM) according to the WHO classification [51–56]. The histological analysis of hMM was reported using original tissue samples and in situ hybridization [55]. Several MM cell lines using pleural fluid and tissue samples from mesothelioma patients have been established [19,45,57–60] and underwent genetic analysis to search for new ther- apeutic targets. Further, murine models of MM induced by asbestos
Fig. 5. Comparison of gene expression profile between cMM organoids and 2D cells. RNA was extracted from each sample of organoids and 2D cells for RNA sequencing (RNA-seq) analysis. The differential expression of RNA volcano plot in organoids and 2D cells (A). Hierarchical clustering of the top 50 differentially expressed genes in organoids vs 2D cells (B). The left panel shows the heat map of gene expressions in organoids and the right panel shows that of 2D cells. The red color indicates an increase in the expression level, while the blue indicates a decrease. Enriched molecular signatures in cMM organoids (C). RNA-seq data were subjected to Gene Set Enrichment Analysis (GSEA). NES is a normalized enrichment score.
Fig. 6. Comparison of the expression level of cell adhesion-related genes between cMM organoids and 2D cells. Expression of CDH1 (E-cadherin), CDH2 (N-cad- herin), VIM (vimentin), ITGAE, ITGAV, ITGA2, ITGA3, and IITGA6 mRNA in organoids and 2D cells was determined by quantitative real-time PCR (A, n = 3). Expression level of each gene was quantified based on the ratio of expression level to GAPDH. Results were expressed as mean ± S.E.M. *P < 0.05 vs. Organoid. Comparison of protein expression of E-cadherin between cMM organoids and 2D cells. Representative photomicrographs were shown (B, n = 4). Scale bar: 50 μm. Summary of the present study. Mesothelioma cells in pleural effusion have diversity in differentiation. In 2D cultured cells, downregulation of E-cadherin leads to mesenchymal properties, while 3D cultured cells might exhibit epithelial properties through the upregulation of E-cadherin.
exposure [61–63] or genetic engineering using vector viruses [61, 64–67] have also been developed. However, these models could not fully reflect the behavior of the original tumor due to differences in animal species and lack of immunity [69,6168]. Although these models introduced several data about MM, it is still insufficient and there are limitations to applying these models for precision medicine. Thus, developing new patient-derived models will be more relevant and of great interest.
Patient-derived tumor organoid models are 3D culture models that can be grown from adult stem cells and can self-organize into 3D cellular structures, and reflect the characteristics of original tissue [70,71] and inter- and intra-tumor heterogeneity among individuals [72,73]. There are reports on MM 3D cultures as spheroids [23,25,74], as well as organoids [26,27,61]. The similarities between dogs and humans in various tumors and diseases have been cited, and canine tumor research has been proposed as a preclinical model for humans [75]. In the current study, we for the first time established cMM organoids using pleural effusion from different dogs with cMM (Fig. 1), and TEM images showed that these organoids could reflect the fine structure of MM cells (Fig. 1D). Furthermore, with IHC staining, cMM organoids expressed cytokeratin, vimentin, and mesothelioma markers (WT-1 and meso- thelin) (Fig. 3) which were also reported in several human studies [54, 76]. These data indicated that our established cMM organoids main- tained the properties of cMM.
Considering the culture medium for cMM organoids, most studies on hMM have reported that RPMI or DMEM was used for culturing MM 2D cells [45,48,57] and 3D cultures [26,27,57]. Few papers have examined appropriate culture medium contents for MM organoids at present. Kim et al. reported that mesothelioma spheroid formation was not observed in LHC-MM (Lechner and LaVeck medium; Biosource International, Camarillo, CA), a mesothelioma-specific medium [23]. Tsugeno’s group described that the cell proliferation rate of MSTO, a mesothelioma cell line known to overexpress EGFR, was significantly reduced when cultured in serum-free media such as STK1 and STK2 (DS Pharma Biomedical Co., Ltd., Osaka, Japan) compared with DMEM medium [77]. In the present study, we for the first time showed that the suitable medium and supplements necessary for the optimum growth of cMM organoids are advanced DMEM/F12 and 50 % W, N, R, EGF, and FGF2 (Fig. 2, Table 1). This suggests that Wnt, EGFR, and FGFR signaling probably have important roles in cMM organoid growth and thus their targeting may help in the treatment of cMM. The EGFR was shown frequently overexpressed in the majority of hMM patients [78–80] and was therapeutically targeted [81,82]. Besides, some studies have sug- gested that expression of FGFR is involved in hMM and drug resistance [83,84] and that there are hMM cell lines with high co-expression of FGFR1 and FGF2 [83,85]. In addition, overexpression of FGFs and FGFRs was also identified as signaling molecules driving malignant growth in MPM, which leads to several studies of therapeutic attempts targeting FGF2-FGFR [83–86]. From these reports and present results, cMM organoid medium was supposed to be applied also to hMM orga- noid culture.
Despite the advances in cancer therapies during the last decades, resistance to traditional chemotherapeutic agents and novel targeted drugs is still the main challenge [87–89]. Certain studies have reported that hMM cells cultured in 3D could acquire a property termed multi- cellular resistance meaning spheroids or organoids tend to resist apoptosis or drugs, which mimics the chemoresistance observed in vivo and can effectively recapitulate some of the complexity of solid tumors [25,90,91]. In the present study, cMM organoids showed resistance against most anti-cancer drugs than 2D cells, while both organoids and 2D cells were relatively sensitive to cisplatin (Fig. 4B). Since the sensi- tivity of organoids to drug screening is similar to the response of patients to drugs, our established cMM organoids may be useful for precision medicine purposes in cMM disease.
The potentials of tumor progression and metastasis were shown to be correlated with alterations in the adhesion characteristics of neoplastic
cells allowing them to lose epithelial integrity and polarity, intra- vasation, and survival into the bloodstream, as well as colonization in other organs [92]. Some reports showed that the cytological analysis of the pleural fluids generally reveals the presence of free spheroidal ag- gregates of hMM cells, which are strongly linked to malignancy, sug- gesting that they have the propensity to survive in the pleural fluid [93, 94]. The levels of serum and pleural fluid soluble cell adhesion mole- cules, E-cadherin, and intercellular adhesion molecule-1 were shown significantly elevated in hMM patients compared with healthy controls [95,96] and necessary for efficient adhesion and disease progression[96,97]. It was also stated that high E-cadherin expression in hMM correlates with therapeutic resistance [98]. In our present study, cMM cells expressed E-cadherin higher in organoids than in 2D cells (Fig. 6A, B). Considering our data, cMM organoids probably maintain behavior and properties in the pleural fluid and keep their malignancy more than 2D cells (Fig. 6C).
5. Conclusion
We for the first time generated cMM organoids from pleural effusion samples. The cMM organoids showed a spherical or luminal morphology, expressed mesothelioma markers, and demonstrated high cell adhesion properties. It was revealed that EGF, FGF2, and WNR were necessary for the optimum growth of cMM organoids. The cMM orga- noids showed more resistance against several anti-cancer drugs and differential gene expression profiles than their corresponding 2D cells. These data suggest that our established cMM organoids may reflect the original tumor histology and could be available as a novel experimental model for MM to provide new insights for human and canine MM treatment. Our 3D cMM organoid model would have potential as a new experimental tool, which could be applied to compare the unique dif- ferences in two- or three-dimensional behavior of mesotheliomas, or to study the expressivity of adhesion molecules and tumor dynamics.
CRediT authorship contribution statement
All authors have approved submission of the manuscript and declare that no conflict of interest exists. Mohamed Elbadawy, Yomogi Sato, Haru Yamamoto, Yusuke Ishihara, Rina Nabeta and Amira Abugomaa carried out the experiment. Yomogi Sato Kazuhiko Suzuki and Yusuke Ishihara performed pathological experiments. Ryouichi Tsunedomi and Hiroaki Nagano performed RNA sequencing analysis. Daigo Azakami, Tsuyoshi Uchide and Ryuji Fukushima informed owners and collected samples from diseased dogs. Yuta Shinohara and Masahiro Kaneda provided experimental tool and resorce. Hideyuki Yamawaki revised manuscript. Tatsuya Usui and Kazuaki Sasaki designed study, analyzed, and interpreted the data, and wrote manuscript.
Conflict of Interest Statement
The authors declare no competing financial and non-financial interests.
Data Availability
Data will be made available on request.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2023.114651.
References
[1] K. Kawanishi, Diverse properties of the mesothelial cells in health and disease, Pleura Perito 1 (2) (2016) 79–89.
- [2] E. Hiriart, R. Deepe, A. Wessels, Mesothelium and malignant mesothelioma, J. Dev. Biol. 7 (2) (2019).
- [3] R. Tranchant, F. Montagne, M.C. Jaurand, D. Jean, Molecular heterogeneity of malignant pleural mesotheliomas, Bull. du Cancer 105 (1) (2018) 35–45.
- [4] J.L. Sauter, S. Dacic, F. Galateau-Salle, R.L. Attanoos, K.J. Butnor, A. Churg, A. N. Husain, K. Kadota, A. Khoor, A.G. Nicholson, V. Roggli, F. Schmitt, M.-S. Tsao, W.D. Travis, The 2021 WHO classification of tumors of the pleura: advances since the 2015 classification, J. Thorac. Oncol. 17 (5) (2022) 608–622.
- [5] S. Dacic, Pleural mesothelioma classification—update and challenges, Mod. Pathol. 35 (1) (2022) 51–56.
- [6] M. Elbadawy, T. Usui, T. Mori, R. Tsunedomi, S. Hazama, R. Nabeta, T. Uchide, R. Fukushima, T. Yoshida, M. Shibutani, T. Tanaka, S. Masuda, R. Okada,
R. Ichikawa, T. Omatsu, T. Mizutani, Y. Katayama, S. Noguchi, S. Iwai,
T. Nakagawa, Y. Shinohara, M. Kaneda, H. Yamawaki, K. Sasaki, Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture, Cancer Sci. 110 (9) (2019) 2806–2821. - [7] A. Abugomaa, M. Elbadawy, H. Yamamoto, H. Ayame, Y. Ishihara, Y. Sato, H. Yamawaki, M. Kaneda, T. Usui, K. Sasaki, Establishment of a direct 2.5D organoid culture model using companion animal cancer tissues, Biomed. Pharmacother. 154 (2022), 113597.
- [8] M. Elbadawy, K. Fujisaka, H. Yamamoto, R. Tsunedomi, H. Nagano, H. Ayame, Y. Ishihara, T. Mori, D. Azakami, T. Uchide, R. Fukushima, A. Abugomaa,
M. Kaneda, H. Yamawaki, Y. Shinohara, T. Omatsu, T. Mizutani, T. Usui, K. Sasaki, Establishment of an experimental model of normal dog bladder organoid using a three-dimensional culture method, Biomed. Pharmacother. 151 (2022), 113105. - [9] A. Abugomaa, M. Elbadawy, H. Yamawaki, T. Usui, K. Sasaki, Emerging roles of cancer stem cells in bladder cancer progression, tumorigenesis, and resistance to chemotherapy: a potential therapeutic target for bladder cancer, Cells 9 (1) (2020) 235.
- [10] H. Yamamoto, M. Elbadawy, K. Fujisaka, Y. Sato, T. Ohmori, Y. Shinohara,
Y. Hatano, D. Kobayashi, A. Gomyo, Y. Sudo, D. Azakami, T. Uchide, R. Fukushima, S. Morita, A. Abugomaa, H. Yamawaki, M. Kaneda, T. Usui, K. Sasaki, Evaluation of the safety and feasibility of apheresis in dogs: for application in metastatic cancer research, Animals 11 (10) (2021) 2770. - [11] H.L. Moberg, I. Gramer, I. Schofield, L. Blackwood, D. Killick, S.L. Priestnall,
A. Guill ́en, Clinical presentation, treatment and outcome of canine malignant mesothelioma: a retrospective study of 34 cases, Vet. Comp. Oncol. 20 (1) (2022) 304–312. - [12] E. Milne, Y. Martinez Pereira, C. Muir, T. Scase, D.J. Shaw, G. McGregor,
L. Oldroyd, E. Scurrell, M. Martin, C. Devine, H. Hodgkiss-Geere, Immunohistochemical differentiation of reactive from malignant mesothelium as a diagnostic aid in canine pericardial disease, J. Small Anim. Pract. 59 (5) (2018) 261–271. - [13] J.H. Kim, Y.K. Choi, H.Y. Yoon, O.K. Kweon, D.Y. Kim, Juvenile malignant mesothelioma in a dog, J. Vet. Med. Sci. 64 (3) (2002) 269–271.
- [14] S.A. Vural, Z. Ozyildiz, S.Y. Ozsoy, Pleural mesothelioma in a nine-month-old dog, Ir. Vet. J. 60 (1) (2007) 30–33.
- [15] A.L. Hudson, C. Weir, E. Moon, R. Harvie, S. Klebe, S.J. Clarke, N. Pavlakis, V. M. Howell, Establishing a panel of chemo-resistant mesothelioma models for investigating chemo-resistance and identifying new treatments for mesothelioma, Sci. Rep. 4 (2014) 6152.
- [16] A.S. Moore, C. Kirk, A. Cardona, Intracavitary cisplatin chemotherapy experience with six dogs, J. Vet. Intern. Med. 5 (4) (1991) 227–231.
- [17] E.P. Spugnini, S. Crispi, A. Scarabello, G. Caruso, G. Citro, A. Baldi, Piroxicam and intracavitary platinum-based chemotherapy for the treatment of advanced mesothelioma in pets: preliminary observations, J. Exp. Clin. Cancer Res.: CR 27 (1) (2008) 6.
- [18] K.W. Seo, U.S. Choi, Y.C. Jung, S.J. Hong, Y.E. Byeun, M.S. Kang, B. Pachrin, W. H. Kim, C.Y. Hwang, D.Y. Kim, H.Y. Youn, C.W. Lee, Palliative intravenous cisplatin treatment for concurrent peritoneal and pleural mesothelioma in a dog, J. Vet. Med. Sci. 69 (2) (2007) 201–204.
- [19] D.J. Colin, D. Cottet-Dumoulin, A. Faivre, S. Germain, F. Triponez, V. Serre-Beinier, Experimental model of human malignant mesothelioma in athymic mice, Int. J. Mol. Sci. 19 (7) (2018).
- [20] K.P. Seastedt, N. Pruett, C.D. Hoang, Mouse models for mesothelioma drug discovery and development, Expert Opin. Drug Discov. 16 (6) (2021) 697–708.
- [21] T. Chernova, X.M. Sun, I.R. Powley, S. Galavotti, S. Grosso, F.A. Murphy, G.
J. Miles, L. Cresswell, A.V. Antonov, J. Bennett, A. Nakas, D. Dinsdale, K. Cain, M. Bushell, A.E. Willis, M. MacFarlane, Molecular profiling reveals primary mesothelioma cell lines recapitulate human disease, Cell Death Differ. 23 (7) (2016) 1152–1164. - [22] G. Cramer, M. Shin, S. Hagan, S.I. Katz, C.B. Simone 2nd, T.M. Busch, K.A. Cengel, Modeling epidermal growth factor inhibitor-mediated enhancement of photodynamic therapy efficacy using 3D mesothelioma cell culture, Photochem. Photobiol. 95 (1) (2019) 397–405.
- [23] K.U. Kim, S.M. Wilson, K.S. Abayasiriwardana, R. Collins, L. Fjellbirkeland, Z. Xu, D.M. Jablons, S.L. Nishimura, V.C. Broaddus, A novel in vitro model of human mesothelioma for studying tumor biology and apoptotic resistance, Am. J. Respir. Cell Mol. Biol. 33 (6) (2005) 541–548.
- [24] X. Xiang, Y. Phung, M. Feng, K. Nagashima, J. Zhang, V.C. Broaddus, R. Hassan, D. Fitzgerald, M. Ho, The development and characterization of a human mesothelioma in vitro 3D model to investigate immunotoxin therapy, PloS One 6 (1) (2011), e14640.
- [25] D. Barbone, L. Van Dam, C. Follo, P.V. Jithesh, S.D. Zhang, W.G. Richards,
R. Bueno, D.A. Fennell, V.C. Broaddus, Analysis of gene expression in 3D spheroids
Biomedicine & Pharmacotherapy 162 (2023) 114651
highlights a survival role for ASS1 in mesothelioma, PloS One 11 (3) (2016),
e0150044.
- [26] A.R. Mazzocchi, S.A.P. Rajan, K.I. Votanopoulos, A.R. Hall, A. Skardal, In vitro
patient-derived 3D mesothelioma tumor organoids facilitate patient-centric
therapeutic screening, Sci. Rep. 8 (1) (2018) 2886.
- [27] Y. Gao, M. Kruithof-de Julio, R.-W. Peng, P. Dorn, Organoids as a Model for
Precision Medicine in Malignant Pleural Mesothelioma: Where Are We Today?,
Cancers, 2022.
- [28] O. Zeira, E. Ghezzi, L. Pettinari, V. Re, D.M. Lupi, S.L. Benali, S. Borgonovo,
G. Alessandri, F. Petrella, R. Paroni, M. Dei Cas, C. Tremolada, V. Cocc`e, A. Pessina, Case report: microfragmented adipose tissue drug delivery in canine mesothelioma: a case report on safety, feasibility, and clinical findings, Front. Vet. Sci. 7 (2020), 585427.
- [29] J.M. Closa, A. Font, J. Mascort, Pericardial mesothelioma in a dog: long-term survival after pericardiectomy in combination with chemotherapy, J. Small Anim. Pract. 40 (8) (1999) 383–386.
- [30] S. Lapp, V.M. Pfankuche, W. Baumg ̈artner, C. Puff, Viral oncolysis – can insights from measles be transferred to canine distemper virus? Viruses 6 (6) (2014) 2340–2375.
- [31] J. Wang, X. Li, H. Chen, Organoid models in lung regeneration and cancer, Cancer Lett. 475 (2020) 129–135.
- [32] A. Abugomaa, M. Elbadawy, Patient-derived organoid analysis of drug resistance in precision medicine: is there a value? Expert Rev. Precision Med. Drug Dev. 5 (1) (2020) 1–5.
- [33] M. Elbadawy, M. Yamanaka, Y. Goto, K. Hayashi, R. Tsunedomi, S. Hazama,
H. Nagano, T. Yoshida, M. Shibutani, R. Ichikawa, J. Nakahara, T. Omatsu,
T. Mizutani, Y. Katayama, Y. Shinohara, A. Abugomaa, M. Kaneda, H. Yamawaki, T. Usui, K. Sasaki, Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model, Biomaterials 237 (2020), 119823. - [34] M. Elbadawy, Y. Kato, N. Saito, K. Hayashi, A. Abugomaa, M. Kobayashi,
T. Yoshida, M. Shibutani, M. Kaneda, H. Yamawaki, T. Mizutani, C.-K. Lim,
M. Saijo, K. Sasaki, T. Usui, T. Omatsu, Establishment of intestinal organoid from rousettus leschenaultii and the susceptibility to bat-associated viruses, SARS-CoV-2 and Pteropine Orthoreovirus, Int. J. Mol. Sci. 22 (19) (2021) 10763. - [35] T. Yoshida, M. Kobayashi, S. Uomoto, K. Ohshima, E. Hara, Y. Katoh, N. Takahashi, T. Harada, T. Usui, M. Elbadawy, M. Shibutani, The potential of organoids in toxicologic pathology: role of toxicologic pathologists in in vitro chemical hepatotoxicity assessment, J. Toxicol. Pathol. 35 (3) (2022) 225–235.
- [36] M. Elbadawy, A. Abugomaa, H. Yamawaki, T. Usui, K. Sasaki, Development of prostate cancer organoid culture models in basic medicine and translational research, Cancers 12 (4) (2020) 777.
- [37] M. Elbadawy, T. Usui, H. Yamawaki, K. Sasaki, Development of an experimental model for analyzing drug resistance in colorectal cancer, Cancers 10 (6) (2018) 164.
- [38] T. Usui, M. Sakurai, K. Umata, M. Elbadawy, T. Ohama, H. Yamawaki, S. Hazama, H. Takenouchi, M. Nakajima, R. Tsunedomi, N. Suzuki, H. Nagano, K. Sato,
M. Kaneda, K. Sasaki, Hedgehog signals mediate anti-cancer drug resistance in three-dimensional primary colorectal cancer organoid culture, Int. J. Mol. Sci. 19 (4) (2018) 1098. - [39] A. Abugomaa, M. Elbadawy, M. Yamanaka, Y. Goto, K. Hayashi, T. Mori, T. Uchide, D. Azakami, R. Fukushima, T. Yoshida, M. Shibutani, R. Yamashita, M. Kobayashi, H. Yamawaki, Y. Shinohara, M. Kaneda, T. Usui, K. Sasaki, Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture, Sci. Rep. 10 (1) (2020) 9393.
- [40] M. Elbadawy, Y. Sato, T. Mori, Y. Goto, K. Hayashi, M. Yamanaka, D. Azakami, T. Uchide, R. Fukushima, T. Yoshida, M. Shibutani, M. Kobayashi, Y. Shinohara, A. Abugomaa, M. Kaneda, H. Yamawaki, T. Usui, K. Sasaki, Anti-tumor effect of trametinib in bladder cancer organoid and the underlying mechanism, Cancer Biol. Ther. 22 (5–6) (2021) 357–371.
- [41] M. Elbadawy, K. Hayashi, H. Ayame, Y. Ishihara, A. Abugomaa, M. Shibutani, S.- M. Hayashi, S. Hazama, H. Takenouchi, M. Nakajima, R. Tsunedomi, N. Suzuki, H. Nagano, Y. Shinohara, M. Kaneda, H. Yamawaki, T. Usui, K. Sasaki, Anti-cancer activity of amorphous curcumin preparation in patient-derived colorectal cancer organoids, Biomed. Pharmacother. 142 (2021), 112043.
- [42] R.K. Pramod, A. Mitra, In vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus), J. Assist. Reprod. Genet. 31 (8) (2014) 993–1001.
- [43] A.N. Husain, T.V. Colby, N.G. Ordo ́n ̃ez, T.C. Allen, R.L. Attanoos, M.B. Beasley, K. J. Butnor, L.R. Chirieac, A.M. Churg, S. Dacic, F. Galateau-Sall ́e, A. Gibbs, A.
M. Gown, T. Krausz, L.A. Litzky, A. Marchevsky, A.G. Nicholson, V.L. Roggli, A. K. Sharma, W.D. Travis, A.E. Walts, M.R. Wick, Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group, Arch. Pathol. Lab. Med. 142 (1) (2018) 89–108. - [44] A.J. Moore, R.J. Parker, J. Wiggins, Malignant mesothelioma, Orphanet J. Rare Dis. 3 (2008) 34.
- [45] V. Relan, L. Morrison, K. Parsonson, B.E. Clarke, E.E. Duhig, M.N. Windsor, K.
S. Matar, R. Naidoo, L. Passmore, E. McCaul, D. Courtney, I.A. Yang, K.M. Fong, R. V. Bowman, Phenotypes and karyotypes of human malignant mesothelioma cell lines, PloS One 8 (3) (2013), e58132. - [46] M. Nishiyama, R. Tsunedomi, K. Yoshimura, N. Hashimoto, S. Matsukuma,
H. Ogihara, S. Kanekiyo, M. Iida, K. Sakamoto, N. Suzuki, S. Takeda, S. Yamamoto, S. Yoshino, T. Ueno, Y. Hamamoto, S. Hazama, H. Nagano, Metastatic ability and the epithelial-mesenchymal transition in induced cancer stem-like hepatoma cells, Cancer Sci. 109 (4) (2018) 1101–1109. - [47] A. Subramanian, P. Tamayo, V.K. Mootha, S. Mukherjee, B.L. Ebert, M.A. Gillette, A. Paulovich, S.L. Pomeroy, T.R. Golub, E.S. Lander, J.P. Mesirov, Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles, Proc. Natl. Acad. Sci. U. S. A. 102 (43) (2005) 15545–15550.
- [48] D. Horio, T. Minami, H. Kitai, H. Ishigaki, Y. Higashiguchi, N. Kondo, S. Hirota, K. Kitajima, Y. Nakajima, Y. Koda, E. Fujimoto, Y. Negi, M. Niki, S. Kanemura, E. Shibata, K. Mikami, R. Takahashi, T. Yokoi, K. Kuribayashi, T. Kijima, Tumor- associated macrophage-derived inflammatory cytokine enhances malignant potential of malignant pleural mesothelioma, Cancer Sci. 111 (8) (2020) 2895–2906.
- [49] T. Usui, M. Sakurai, S. Nishikawa, K. Umata, Y. Nemoto, T. Haraguchi, K. Itamoto, T. Mizuno, S. Noguchi, T. Mori, S. Iwai, T. Nakagawa, H. Yamawaki, T. Ohama, K. Sato, Establishment of a dog primary prostate cancer organoid using the urine cancer stem cells, Cancer Sci. 108 (12) (2017) 2383–2392.
- [50] T. Usui, M. Sakurai, S. Enjoji, H. Kawasaki, K. Umata, T. Ohama, N. Fujiwara,
R. Yabe, S. Tsuji, H. Yamawaki, S. Hazama, H. Takenouchi, M. Nakajima,
R. Tsunedomi, N. Suzuki, H. Nagano, K. Sato, Establishment of a novel model for anticancer drug resistance in three-dimensional primary culture of tumor microenvironment, Stem Cells Int. 2016 (2016) 7053872. - [51] W.D. Travis, E. Brambilla, A.G. Nicholson, Y. Yatabe, J.H.M. Austin, M.B. Beasley, L.R. Chirieac, S. Dacic, E. Duhig, D.B. Flieder, K. Geisinger, F.R. Hirsch,
Y. Ishikawa, K.M. Kerr, M. Noguchi, G. Pelosi, C.A. Powell, M.S. Tsao, I. Wistuba, The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification, J. Thorac. Oncol.: Off. Publ. Int. Assoc. Study Lung Cancer 10 (9) (2015) 1243–1260. - [52] M.G. Zauderer, A. Martin, J. Egger, H. Rizvi, M. Offin, A. Rimner, P.S. Adusumilli, V.W. Rusch, M.G. Kris, J.L. Sauter, M. Ladanyi, R. Shen, The use of a next- generation sequencing-derived machine-learning risk-prediction model (OncoCast- MPM) for malignant pleural mesothelioma: a retrospective study, Lancet Digit. Health 3 (9) (2021) e565–e576.
- [53] A.R. D’Angelo, G. Di Francesco, Sclerosing peritoneal mesothelioma in a dog: histopathological, histochemical and immunohistochemical investigations, Vet. Ital. 50 (4) (2014) 301–305.
- [54] N.V. Son, J.K. Chambers, T. Shiga, T.E. Kishimoto, S. Kikuhara, K. Saeki,
R. Fujiwara, M. Tsuboi, R. Nishimura, K. Uchida, H. Nakayama, Sarcomatoid mesothelioma of tunica vaginalis testis in the right scrotum of a dog, J. Vet. Med. Sci. 80 (7) (2018) 1125–1128. - [55] A. Fassina, R. Cappellesso, V. Guzzardo, L. Dalla Via, S. Piccolo, L. Ventura,
M. Fassan, Epithelial-mesenchymal transition in malignant mesothelioma, Mod. Pathol. 25 (1) (2012) 86–99. - [56] A. Schramm, I. Opitz, S. Thies, B. Seifert, H. Moch, W. Weder, A. Soltermann, Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma, Eur. J. Cardio-Thorac. Surg. 37 (3) (2010) 566–572.
- [57] Y. Su, X. Zhang, S. Bidlingmaier, C.R. Behrens, N.K. Lee, B. Liu, ALPPL2 is a highly specific and targetable tumor cell surface antigen, Cancer Res. 80 (20) (2020) 4552–4564.
- [58] L.S. Manning, D. Whitaker, A.R. Murch, M.J. Garlepp, M.R. Davis, A.W. Musk, B. W. Robinson, Establishment and characterization of five human malignant mesothelioma cell lines derived from pleural effusions, Int. J. Cancer 47 (2) (1991) 285–290.
- [59] N. Usami, T. Fukui, M. Kondo, T. Taniguchi, T. Yokoyama, S. Mori, K. Yokoi,
Y. Horio, K. Shimokata, Y. Sekido, T. Hida, Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients, Cancer Sci. 97 (5) (2006) 387–394. - [60] M.M. Philippeaux, J.C. Pache, S. Dahoun, M. Barnet, J.H. Robert, J. Mau ̈el, A. Spiliopoulos, Establishment of permanent cell lines purified from human mesothelioma: morphological aspects, new marker expression and karyotypic analysis, Histochem. Cell Biol. 122 (3) (2004) 249–260.
- [61] M. Shamseddin, J. Obacz, M.J. Garnett, R.C. Rintoul, H.E. Francies, S.J. Marciniak, Use of preclinical models for malignant pleural mesothelioma, Thorax 76 (11) (2021) 1154–1162.
- [62] T. Chernova, F.A. Murphy, S. Galavotti, X.M. Sun, I.R. Powley, S. Grosso,
A. Schinwald, J. Zacarias-Cabeza, K.M. Dudek, D. Dinsdale, J. Le Quesne,
J. Bennett, A. Nakas, P. Greaves, C.A. Poland, K. Donaldson, M. Bushell, A.E. Willis, M. MacFarlane, Long-fiber carbon nanotubes replicate asbestos-induced mesothelioma with disruption of the tumor suppressor gene Cdkn2a (Ink4a/Arf), Curr. Biol.: CB 27 (21) (2017) 3302–3314, e6. - [63] M. Suzui, M. Futakuchi, K. Fukamachi, T. Numano, M. Abdelgied, S. Takahashi, M. Ohnishi, T. Omori, S. Tsuruoka, A. Hirose, J. Kanno, Y. Sakamoto, D.
B. Alexander, W.T. Alexander, X. Jiegou, H. Tsuda, Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors, Cancer Sci. 107 (7) (2016) 924–935. - [64] J. Jongsma, E. van Montfort, M. Vooijs, J. Zevenhoven, P. Krimpenfort, M. van der Valk, M. van de Vijver, A. Berns, A conditional mouse model for malignant mesothelioma, Cancer Cell 13 (3) (2008) 261–271.
- [65] A.M. Kukuyan, E. Sementino, Y. Kadariya, C.W. Menges, M. Cheung, Y. Tan, K. Q. Cai, M.J. Slifker, S. Peri, A.J. Klein-Szanto, F.J. Rauscher 3rd, J.R. Testa, Inactivation of Bap1 cooperates with losses of Nf2 and Cdkn2a to drive the development of pleural malignant mesothelioma in conditional mouse models, Cancer Res. 79 (16) (2019) 4113–4123.
- [66] J. Badhai, G.K. Pandey, J.Y. Song, O. Krijgsman, R. Bhaskaran, G. Chandrasekaran, M.C. Kwon, L. Bombardelli, K. Monkhorst, C. Grasso, J. Zevenhoven, J. van der Vliet, M. Cozijnsen, P. Krimpenfort, D. Peeper, M. van Lohuizen, A. Berns, Combined deletion of Bap1, Nf2, and Cdkn2ab causes rapid onset of malignant mesothelioma in mice, J. Exp. Med. 217 (6) (2020).
Biomedicine & Pharmacotherapy 162 (2023) 114651
- [67] E. Sementino, C.W. Menges, Y. Kadariya, S. Peri, J. Xu, Z. Liu, R.G. Wilkes, K.
Q. Cai, F.J. Rauscher 3rd, A.J. Klein-Szanto, J.R. Testa, Inactivation of Tp53 and Pten drives rapid development of pleural and peritoneal malignant mesotheliomas, J. Cell. Physiol. 233 (11) (2018) 8952–8961. - [68] L. Wu, G. Allo, T. John, M. Li, T. Tagawa, I. Opitz, M. Anraku, Z. Yun, M. Pintilie, B. Pitcher, G. Liu, R. Feld, M.R. Johnston, M. de Perrot, M.S. Tsao, Patient-Derived Xenograft establishment from human malignant pleural mesothelioma, Clin. Cancer Res. 23 (4) (2017) 1060–1067.
- [69] N. Kalra, J. Zhang, A. Thomas, L. Xi, M. Cheung, J. Talarchek, S. Burkett, M.
G. Tsokos, Y. Chen, M. Raffeld, M. Miettinen, I. Pastan, J.R. Testa, R. Hassan, Mesothelioma patient derived tumor xenografts with defined BAP1 mutations that mimic the molecular characteristics of human malignant mesothelioma, BMC Cancer 15 (2015) 376. - [70] M. Bleijs, M. van de Wetering, H. Clevers, J. Drost, Xenograft and organoid model systems in cancer research, EMBO J. 38 (15) (2019), e101654.
- [71] H. Clevers, Modeling development and disease with organoids, Cell 165 (7) (2016) 1586–1597.
- [72] L. Huang, A. Holtzinger, I. Jagan, M. BeGora, I. Lohse, N. Ngai, C. Nostro, R. Wang, L.B. Muthuswamy, H.C. Crawford, C. Arrowsmith, S.E. Kalloger, D.J. Renouf, A. A. Connor, S. Cleary, D.F. Schaeffer, M. Roehrl, M.S. Tsao, S. Gallinger, G. Keller, S. K. Muthuswamy, Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids, Nat. Med. 21 (11) (2015) 1364–1371.
- [73] M. van de Wetering, H.E. Francies, J.M. Francis, G. Bounova, F. Iorio, A. Pronk, W. van Houdt, J. van Gorp, A. Taylor-Weiner, L. Kester, A. McLaren-Douglas,
J. Blokker, S. Jaksani, S. Bartfeld, R. Volckman, P. van Sluis, V.S. Li, S. Seepo, C. Sekhar Pedamallu, K. Cibulskis, S.L. Carter, A. McKenna, M.S. Lawrence,L. Lichtenstein, C. Stewart, J. Koster, R. Versteeg, A. van Oudenaarden, J. Saez- Rodriguez, R.G. Vries, G. Getz, L. Wessels, M.R. Stratton, U. McDermott,
M. Meyerson, M.J. Garnett, H. Clevers, Prospective derivation of a living organoid biobank of colorectal cancer patients, Cell 161 (4) (2015) 933–945. - [74] H. Kim, Y. Phung, M. Ho, Changes in global gene expression associated with 3D structure of tumors: an ex vivo matrix-free mesothelioma spheroid model, PloS One 7 (6) (2012), e39556.
- [75] G. Ranieri, C.D. Gadaleta, R. Patruno, N. Zizzo, M.G. Daidone, M.G. Hansson,
A. Paradiso, D. Ribatti, A model of study for human cancer: Spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies, Crit. Rev. Oncol. /Hematol. 88 (1) (2013) 187–197. - [76] S. Dahiya, M. Singh, S. Jain, B. Khuraijam, N. Suroya, S. Mandal, Cytological diagnosis of malignant mesothelioma: a case series, J. Cytol. 39 (3) (2022) 105–109.
- [77] Y. Tsugeno, F. Sato, Y. Muragaki, Y. Kato, Cell culture of human gingival fibroblasts, oral cancer cells and mesothelioma cells with serum-free media, STK1 and STK2, Biomed. Rep. 2 (5) (2014) 644–648.
- [78] A. Destro, G.L. Ceresoli, M. Falleni, P.A. Zucali, E. Morenghi, P. Bianchi,
C. Pellegrini, N. Cordani, V. Vaira, M. Alloisio, A. Rizzi, S. Bosari, M. Roncalli, EGFR overexpression in malignant pleural mesothelioma, Immunohistochem. Mol. Study Clin. – Pathol. Correl., Lung Cancer (Amst., Neth.) 51 (2) (2006) 207–215. - [79] K. Okuda, H. Sasaki, O. Kawano, H. Yukiue, T. Yokoyama, M. Yano, Y. Fujii, Epidermal growth factor receptor gene mutation, amplification and protein expression in malignant pleural mesothelioma, J. Cancer Res. Clin. Oncol. 134 (10) (2008) 1105–1111.
- [80] O. Rena, L.R. Boldorini, E. Gaudino, C. Casadio, Epidermal growth factor receptor overexpression in malignant pleural mesothelioma: prognostic correlations,
J. Surg. Oncol. 104 (6) (2011) 701–705. - [81] S. Dubey, P.A. Ja ̈nne, L. Krug, H. Pang, X. Wang, R. Heinze, C. Watt, J. Crawford, R. Kratzke, E. Vokes, et al., A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307, J. Thorac. Oncol. 5 (10) (2010) 1655–1661.
- [82] P.L. Chia, S. Parakh, M.S. Tsao, N.A. Pham, H.K. Gan, D. Cao, I.J.G. Burvenich, A. Rigopoulos, E.B. Reilly, T. John, A.M. Scott, Targeting and efficacy of Novel mAb806-antibody-drug conjugates in malignant mesothelioma, Pharmaceuticals (Basel, Switzerland) 13 (10) (2020).
- [83] C. Blackwell, C. Sherk, M. Fricko, G. Ganji, M. Barnette, B. Hoang, J. Tunstead, T. Skedzielewski, H. Alsaid, B.M. Jucker, E. Minthorn, R. Kumar, M.P. DeYoung, Inhibition of FGF/FGFR autocrine signaling in mesothelioma with the FGF ligand trap, FP-1039/GSK3052230, Oncotarget 7 (26) (2016) 39861–39871.
- [84] G. Vlacic, M.A. Hoda, T. Klikovits, K. Sinn, E. Gschwandtner, K. Mohorcic,
K. Schelch, C. Pirker, B. Peter-Vo ̈ro ̈smarty, J. Brankovic, B. Dome, V. Laszlo,
T. Cufer, A. Rozman, W. Klepetko, B. Grasl-Kraupp, B. Hegedus, W. Berger, I. Kern, M. Grusch, Expression of FGFR1-4 in malignant pleural mesothelioma tissue and corresponding cell lines and its relationship to patient survival and FGFR inhibitor sensitivity, Cells 8 (9) (2019). - [85] L.A. Marek, T.K. Hinz, A. von M ̈assenhausen, K.A. Olszewski, E.K. Kleczko,
D. Boehm, M.C. Weiser-Evans, R.A. Nemenoff, H. Hoffmann, A. Warth, J.M. Gozgit, S. Perner, L.E. Heasley, Nonamplified FGFR1 is a growth driver in malignant pleural mesothelioma, Mol. Cancer Res.: MCR 12 (10) (2014) 1460–1469. - [86] K. Schelch, M.B. Kirschner, M. Williams, Y.Y. Cheng, N. van Zandwijk, M. Grusch, G. Reid, A link between the fibroblast growth factor axis and the miR-16 family reveals potential new treatment combinations in mesothelioma, Mol. Oncol. 12 (1) (2018) 58–73.
- [87] A. Elfadadny, H.M. El-Husseiny, A. Abugomaa, R.F. Ragab, E.A. Mady,
M. Aboubakr, H. Samir, A.S. Mandour, A. El-Mleeh, A.H. El-Far, A.H. Abd El-Aziz, M. Elbadawy, Role of multidrug resistance-associated proteins in cancer therapeutics: past, present, and future perspectives, Environ. Sci. Pollut. Res. 28 (36) (2021) 49447–49466. - [88] M. Elbadawy, T. Usui, H. Yamawaki, K. Sasaki, Emerging roles of C-Myc in cancer stem cell-related signaling and resistance to cancer chemotherapy: a potential therapeutic target against colorectal cancer, Int. J. Mol. Sci. 20 (9) (2019) 2340.
- [89] M. Elbadawy, T. Usui, H. Yamawaki, K. Sasaki, Novel functions of death-associated protein kinases through mitogen-activated protein kinase-related signals, Int. J. Mol. Sci. 19 (10) (2018) 3031.
- [90] D. Barbone, T.M. Yang, J.R. Morgan, G. Gaudino, V.C. Broaddus, Mammalian target of rapamycin contributes to the acquired apoptotic resistance of human mesothelioma multicellular spheroids, J. Biol. Chem. 283 (19) (2008) 13021–13030.
- [91] D. Barbone, J.A. Ryan, N. Kolhatkar, A.D. Chacko, D.M. Jablons, D.J. Sugarbaker, R. Bueno, A.G. Letai, L.M. Coussens, D.A. Fennell, V.C. Broaddus, The Bcl-2 repertoire of mesothelioma spheroids underlies acquired apoptotic multicellular resistance, Cell Death Dis. 2 (6) (2011), e174.
- [92] N. Makrilia, A. Kollias, L. Manolopoulos, K. Syrigos, Cell adhesion molecules: role and clinical significance in cancer, Cancer Investig. 27 (10) (2009) 1023–1037.
- [93] J. Daubriac, J. Fleury-Feith, L. Kheuang, J. Galipon, A. Saint-Albin, A. Renier, M. Giovannini, F. Galateau-Sall ́e, M.C. Jaurand, Malignant pleural mesothelioma cells resist anoikis as quiescent pluricellular aggregates, Cell Death Differ. 16 (8) (2009) 1146–1155.
Biomedicine & Pharmacotherapy 162 (2023) 114651
- [94] B. Davidson, Biological characteristics of cancers involving the serosal cavities, Crit. Rev. Oncog. 13 (3) (2007) 189–227.
- [95] M. Piazzi, S. Kojic, C. Capanni, N. Stamenkovic, A. Bavelloni, O. Marin, G. Lattanzi, W. Blalock, V. Cenni, Ectopic expression of Ankrd2 affects proliferation, motility and clonogenic potential of human osteosarcoma cells, Cancers (Basel) 13 (2) (2021).
- [96] A. Ito, M. Hagiyama, T. Mimura, M. Matsumoto, T. Wakayama, S. Iseki,
H. Yokozaki, M. Okada, Expression of cell adhesion molecule 1 in malignant pleural mesothelioma as a cause of efficient adhesion and growth on mesothelium, Lab. Investig. 88 (5) (2008) 504–514. - [97] S. Tsagkouli, I.G. Kyriakoulis, K.G. Kyriakoulis, E. Fyta, A. Syrigos, P. Bakakos, A. Charpidou, E. Kotteas, Serum and pleural soluble cell adhesion molecules in mesothelioma patients: a retrospective cohort study, Cancers (Basel) 14 (12) (2022).
- [98] M.L. Yuen, L. Zhuang, E.M. Rath, T. Yu, B. Johnson, K.H. Sarun, Y. Wang, S. Kao, A. Linton, C.J. Clarke, B.C. McCaughan, K. Takahashi, K. Lee, Y.Y. Cheng, The role of E-Cadherin and microRNA on FAK inhibitor response in malignant pleural mesothelioma (MPM), Int. J. Mol. Sci. 22 (19) (2021).